New joint asthma guideline: implications for children and young people
Viv Marsh
Viv Marsh
RN, QN
Independent Prescriber
Clinical Lead for Children’s Asthma
Transformation, Black Country ICB
In association with Rotherham Respiratory
Practice Nurse 2024;54(5):16-19
What does the future hold for the delivery of asthma care for children and young people as we wait for the final version of BTS/SIGN/NICE guidance, and what challenges does the guideline pose for general practice nurses?
Consultation on the long-awaited joint asthma guideline from the British Thoracic Society, Scottish Intercollegiate Guidelines Network and National Institute for Health and Care Excellence (BTS/SIGN/NICE)1 has now closed and final publication is expected later this year. Undoubtedly the new guideline will support some of the transformations to asthma care that are already taking place and, from listening to the lively discussion in the respiratory world, it appears that the document is being seen in a positive light and will be welcomed. However, what does it mean for children and young people (CYP) with asthma – or who may have asthma – and are there any drawbacks for this age group?
This article seeks to explore what the future could hold for the delivery of asthma care for children and young people, and most importantly, what the implications could be for children, young people and their families with implementation of the guideline.
CURRENT GAPS IN ASTHMA CARE FOR CYP
Outcomes for CYP with asthma in the UK are among the poorest in the world and mortality rates for 10–24-year-olds are the highest in Europe.2 You may feel that this is truly shocking given that the UK is a leading first world country with a reputable health service; do hold that thought as you continue to read this article.
Many issues impacting asthma outcomes for CYP have been known and well documented for years. These include poor perceptions of asthma among professionals, as well as children, young people and their families, low levels of confidence for managing asthma in younger age groups in primary care, and poor self-management skills.3 More recently the role of health inequalities has become more widely recognised, with children living in the poorest areas being at the greatest risk of hospital admission due to an asthma attack.4
Narrowing down these issues, there are three clinical areas where huge gaps currently exist for children in the UK. While considering each in turn, the extent to which the new guideline might provide a foundation for improvement will be discussed.
1. NO CLEAR DIAGNOSTIC PROCESS
Current drivers for improving health outcomes across the NHS include early and accurate diagnosis of medical conditions. This is seen clearly in the national bundle of care for children and young people with asthma which points out that unrecognised and untreated asthma leads to dangerous asthma attacks and impairs quality of life for children and their families.5 Asthma is a condition that is both under, and over diagnosed,6 and in a bid to improve diagnostic accuracy the draft joint guideline recommends that diagnosis of asthma should not be confirmed without a clinical history suggestive of asthma and a supporting objective test.
The draft document clarifies that treatment should not be delayed in patients who are acutely unwell and that professionals should be aware that the results of spirometry and Fractional exhaled Nitric Oxide (FeNO) tests may be affected in people being treated with inhaled corticosteroids (ICS).
Because of the variable nature of asthma, diagnosis in patients of any age is difficult as results may be normal at the time of testing, but this does not rule out a diagnosis of asthma. (Tests tend to rule in asthma rather than rule it out, and they may need to be repeated over time.) It is important to recognise that these difficulties are amplified in children who may not be able to perform the preferred objective tests to the required standard; while this is acknowledged by the guideline committee, it would not appear to be fully appreciated and there is no pragmatic guidance to support children, their families or professionals other than to send children for blood tests or refer them for specialist care! As professionals we find the diagnosis of asthma in children both challenging and frustrating for the reasons outlined above. But we must also remember that this is a difficult time for children and their families, and a recent study found that parents felt unsupported and misunderstood during the diagnostic process.7
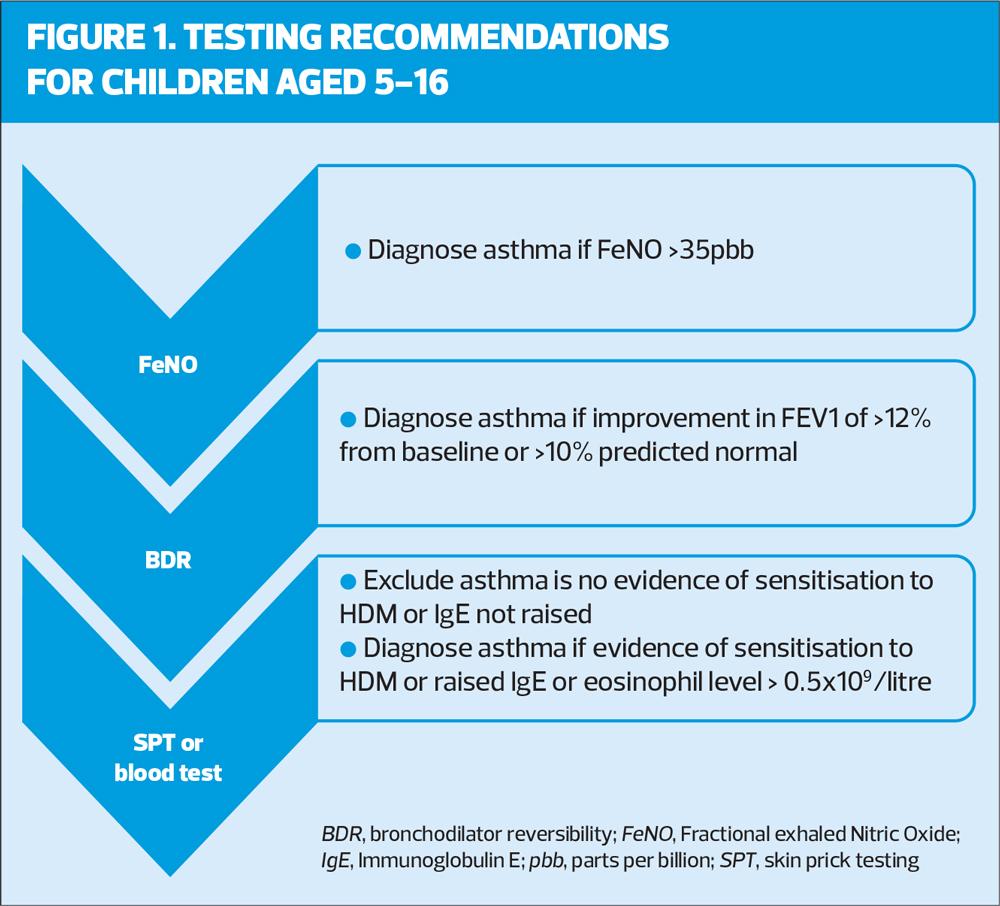
A sequence of testing, based on economic analysis is provided for children from 5 years of age. Tests are seeking evidence of inflammation (airway or blood biomarkers), reversible airflow limitation or allergic sensitisation. The first recommended test is FeNo, a biomarker of inflammation in the airways. If asthma cannot be diagnosed by FeNO (e.g. not available, normal result) the next test is Bronchodilator Reversibility (BDR) using spirometry and if asthma cannot be diagnosed by BDR the next recommended test is either Skin Prick Testing (SPT) or blood testing (Figure 1).
If there are no positive test results but asthma is still suspected the child should be referred to a paediatric respiratory specialist for a second opinion and consideration of a bronchial challenge test.
It will be glaringly obvious to readers that access to the objective tests suggested in the draft guidance, already limited in primary care for adults, is largely unavailable for children. In the short to medium term, an increase in referral for blood tests and for specialist opinion should be expected, which in the author’s opinion is not in the interests of children and their families or of the NHS.
National drivers are calling for improvements not just in diagnostic accuracy but also to diagnose asthma as early as possible.5 The reason for this is so that children can be commenced on the treatment (inhaled corticosteroids, ICS) that will protect them from the impact of uncontrolled symptoms and potentially life-threatening asthma attacks, in addition to supporting healthy lung development and growth reducing the risk of impaired lung function later in life.8
The draft joint guideline recommends that children under 5 can be treated based on clinical judgement and that once they reach 5 years of age, objective tests can be attempted. Children should be observed, managed using clinical judgement and retests should be attempted every 6-12 months ‘until satisfactory results are obtained’. During this period trials of paediatric low dose ICS can be used for periods of 8-12 weeks followed by observed periods without ICS. It is vital that this process is monitored carefully and that parents are well supported, otherwise the risk of asthma attacks will increase.
It is disappointing that the joint guideline does not include a ‘monitored trial of treatment’ for children over 5 years of age. It is currently, and likely to remain for some time, a useful tool to support the diagnosis of asthma in young children.9 The main limitation of using a ‘monitored trial of treatment’ is that all too often it is not administered correctly, rendering it useless for diagnostic purposes. There is an opportunity to directly impact and improve outcomes for children with asthma by providing clear guidance around this process in the final guideline, the question is, will it be taken?
Although the guideline recommendations will be a lever for improving availability and access to tests for all who need them over time, the reality is that for children the benefits are a long way off. So, while on the one hand the call to improve the accuracy of diagnosis with the requirement for objective tests must be seen positively, there is a risk that the guidance could widen existing gaps for children.
2. INCONSISTENT PROVISION OF ESSENTIAL ASTHMA CARE
Evidence-based care for people with a diagnosis of asthma includes regular monitoring, pharmacological and non-pharmacological management, and support for self-management.10 Driven by targets, indicators, capacity issues and poor understanding of the nature of the condition, delivery of asthma care has become routinised in primary care. As a result, personalised care, which is advocated in policy and vital for effectively supporting self-management, can be difficult to achieve in the real world.10
In England, the Quality Outcomes Framework (at the time of writing) requires practices to maintain a register of patients with asthma from 6 years of age. But what about children under 6 years of age? This element of the framework is outdated given that asthma (or suspected asthma) will increasingly be diagnosed before 6 years of age – surely these children should be also be monitored!
The draft joint guideline indicates that all patients with asthma or suspected asthma require monitoring and this may prove to be a lever to ensure children with asthma or suspected asthma have access to the care they need. Monitoring asthma control is highlighted with a recommendation to check:
- Time off work or school due to asthma
- Amount of reliever inhaler used
- Number of courses of oral corticosteroids
- Active or passive exposure to smoking
It is unclear why the use of a symptom questionnaire for children under 12 is not suggested in the draft joint guideline, as it is for adults. This may be due to the lack of consistency and agreement between the tools validated for use in this age group,11 and indeed as a result of limitations in their reliability, Carroll has suggested that a patient-focused consultation has more to offer children with asthma and their families.12
Peak Expiratory Flow measurements are not recommended for monitoring purposes but consideration of FeNO is suggested.
The draft joint guideline includes a strong message about checking inhaler technique stating that patients should be observed using their inhaler device (and spacer where relevant) to check that they can use it properly:
- At every asthma review, either routine or unscheduled
- At every consultation
- When there is deterioration in asthma control
- When the inhaler device is changed
- When the person asks for it to be checked or changed.
Alternatives should be found for anyone who cannot use their device correctly and we must remember here that this includes spacer devices. Many patients, and almost all children under 10 years of age, use pressurised Metered Dose Inhalers (pMDIs) with which a spacer device should always be used to ensure optimal deposition of drug particles in the airways.
In terms of pharmacological management, the draft joint guideline is very clear that short acting beta2 agonists must not be prescribed without concomitant prescription of inhaled corticosteroid for people of any age with asthma. The evidence linking SABA-only treatment to increased asthma risk is well documented, and this guideline will join other key drivers in the call to eradicating this practice. However, there is no guarantee that patients who are prescribed separate ICS-containing and SABA inhalers will use their inhalers correctly as prescribed, and the likelihood of SABA overuse will continue to be a major issue in asthma management until the recent advances outlined below are available to all.
One of the real positives to be taken from the draft joint guideline is the strength of support for greater use of Maintenance and Reliever Therapy (MART) and Anti-Inflammatory Reliever (AIR) treatment approaches. Where patients have a single inhaler for both maintenance and reliever or as needed only reliever treatment, use of beta agonist without ICS should not occur thereby eliminating the risk. It is important to note that patients treated with the MART or AIR approach must not be prescribed separate SABA inhalers.
We must remember that MART and AIR are not the panacea for all because many patients will not want or be able to manage their asthma in this way. Furthermore, current licensing arrangements do not support the use of MART or AIR in children under 12 years of age, although this is likely to change in the near future.
The draft joint guideline pharmacological management recommendations for children aged 5–11 years begin with twice daily paediatric low dose ICS with SABA as needed. Following this, the options are a MART or non-MART pathway. MART can be considered if the child and family are assessed to have the ability to manage a MART regimen. This approach will be off label until MART products licensed for this age group are available.
On the MART pathway, children not controlled on paediatric low dose MART can increase to paediatric moderate dose MART, but in the non-MART pathway the recommendation is to try a leukotriene receptor antagonist (LTRA) initially, if add-on therapy to the twice daily ICS plus as needed SABA is indicated. The appropriateness of this has undoubtably been raised in the guideline consultation process given the risk of neuro-psychiatric adverse effects with LTRA.13
The option to use an ICS/LABA (Long-Acting Beta Agonist) plus SABA as needed is then included, with or without LTRA, and moving from paediatric low dose to paediatric moderate dose if necessary.
Children with ongoing poor asthma control at this point should be referred for specialist care.
Children under 5 years of age with asthma can be treated with paediatric low dose ICS plus as needed SABA titrated to paediatric moderate dose ICS with further addition of LTRA if symptom control cannot be achieved. Beyond this, referral to specialist care is recommended.
3. POOR RECOGNITION OF ASTHMA RISK
‘Complacency in asthma’ was a phrase coined by Levy and colleagues in the report of the National Review of Asthma Deaths.14 This complacency arises from, and drives, poor perceptions of asthma, creating a continuous cycle of sub-optimal care delivery and inadequate self-management skills for those who are most in need. Poor perception of asthma means that asthma is not fully understood or taken seriously, leading to increased risk of morbidity and mortality.
The draft joint guideline suggests that people with asthma who are at risk of poor outcomes should be identified and provided with care that is tailored to their needs. Risk factors include:
- Non-adherence to medication
- Over-use of SABA inhalers
- Repeated episodes of unscheduled care for asthma
It is hoped that the final guideline will be more specific regarding what is considered to be repeated episodes. The national bundle of care for children and young people with asthma5 highlights that children who have had two asthma attacks in 12 months should be regarded as high risk, and this provides a clear steer to primary care to identify and prioritise these children for review.
IN SUMMARY
In light of the fact that this joint guideline is not attempting to be a comprehensive manual guiding asthma management in all situations and contexts, there are broad themes which can be seen in a positive light. The need to improve diagnostic accuracy is supported and potential leverage for improved access to tests such as FeNO acknowledged, however it is disappointing that the recommendations do not relate well to meeting the diagnostic needs of children. A logical framework for providing essential asthma care including monitoring asthma is offered but the pharmacological management options for children are less logical. Risk factors for morbidity and mortality, a major concern in asthma, are recognised in the draft document and greater detail may be incorporated into the final guidance.
On balance, I would argue that the draft guideline lacks focus on children and young people and hope that the consultation responses are taken into consideration before the final guideline is published.
REFERENCES
1. NICE. Asthma: diagnosis, monitoring and chronic asthma management [Draft for Consultation]; 2024 https://www.nice.org.uk/guidance/indevelopment/gid-ng10186/documents
2. The Nuffield Trust International comparisons of health and wellbeing in adolescence and early adulthood; 2019. https://www.nuffieldtrust.org.uk/research/international-comparisons-of-health-and-wellbeing-in-adolescence-and-early-adulthood
3. Kelada L, Molloy CJ, Hibbert P, et al (2021) Child and caregiver experiences and perceptions of asthma self-management. NPJ Prim Care Respir Med 2021;31:42. https://www.nature.com/articles/s41533-021-00253-9
4. Asthma + Lung UK (2023) Breathing Unequal; 2023 https://www.asthmaandlung.org.uk/breathing-unequal#:~:text=Our%20report%20found%20that%20people,die%20from%20their%20lung%20conditions
5. NHS England; National bundle of care for children and young people with asthma;2021 https://www.england.nhs.uk/publication/national-bundle-of-care-for-children-and-young-people-with-asthma/
6. Looijmans-van den Akker I, van Luijn K, Verheij T. Overdiagnosis of asthma in children in primary care: a retrospective analysis. Brit J Gen Pract 2016;66 (644):e152-e157 https://bjgp.org/content/66/644/e152
7. Lange D, Lindenmeyer A, Haroon S, et al. “Will anybody listen?” Parents’ views on childhood asthma care: a qualitative study. BJGP Open 28 May 2024; BJGPO.2024.0070. https://bjgpopen.org/content/early/2024/05/25/BJGPO.2024.0070.long
8. Bui DS, Lodge CJ, Burgess JA, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med 2019; 6(7):535–544
9. Martin J, Townshend J, Brodie M, et al (2022) Diagnosis and management of asthma in children BMJ Paediatr Open. 2022; 6(1): e001277 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9045042/
10. Hodkinson A, Bower P, Grigoroglou C, et al. Self-management interventions to reduce healthcare use and improve quality of life among patients with asthma: a systematic review and network meta-analysis. BMJ 2020;370:m2521. https://www.bmj.com/content/370/bmj.m2521
11. Bousema S, Bohnen AM, Bindels PJ, Elshout G. A systematic review of questionnaires measuring asthma control in children in a primary care population. NPJ Prim Care Respir Med. 2023;33:25 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10336001/
12. Carroll W (2013) Limitations of asthma control questionnaires in the management and follow up of childhood asthma. Paed Respir Rev 2013;14(4): 229-231 https://www.sciencedirect.com/science/article/abs/pii/S1526054213000869
13. MHRA. Montelukast: reminder of the risk of neuropsychiatric reactions; 2024. https://www.gov.uk/drug-safety-update/montelukast-reminder-of-the-risk-of-neuropsychiatric-reactions
14. Royal College of Physicians. Why asthma still kills: National Review of Asthma Deaths; 2014. https://www.rcp.ac.uk/improving-care/resources/why-asthma-still-kills/
Related articles
View all Articles