Protecting patients, protecting the health service: seasonal flu update
Mandy Galloway, Editor
Mandy Galloway, Editor
Practice Nurse 2024;54(5):12-15
With more than ever to do, and less time to do it in, this year’s seasonal flu vaccine campaign is going to be busier than ever – but it is critical that practices aim to boost uptake to protect individuals and reduce winter pressures on the NHS
There are around a billion cases of seasonal influenza annually, including 3–5 million cases of
severe illness.1
In most cases, flu is a minor, self-limiting illness, but it can cause severe illness or death, especially in people at high risk. Globally, it causes 290,000 to 650,000 respiratory deaths annually. Hospitalisation and death due to influenza occur mainly among high-risk groups; conversely, flu can worsen symptoms of other chronic diseases. In severe cases, it can lead to pneumonia and sepsis.1
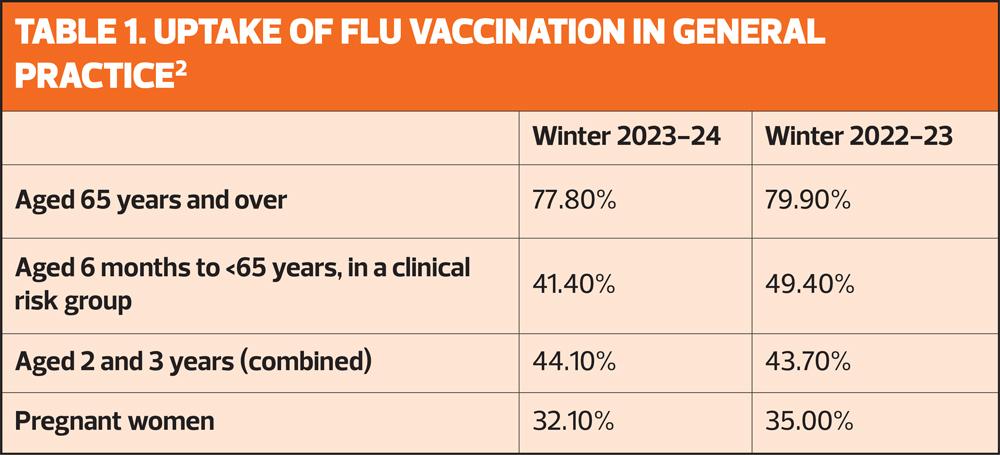
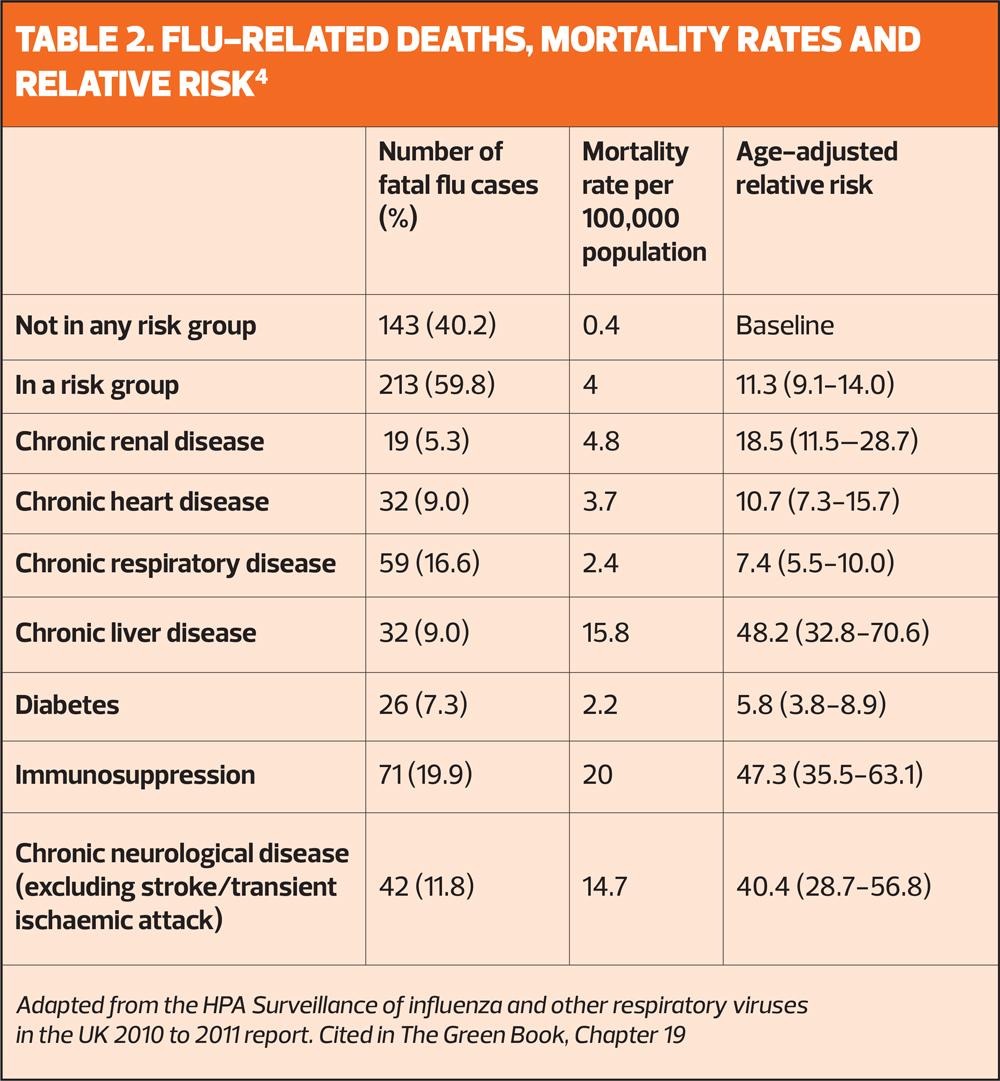
But in the UK, uptake of the seasonal flu vaccine in at risk groups is persistently below target levels, and last winter, only one-in-four people aged 6 months to 65 years in a clinical risk group was vaccinated – down from just below one-in-five the previous year (Table 1).2 The increased risk of death in at risk groups, compared with those who are not in a risk group, is shown in Table 2.
This year’s guidance on the annual seasonal flu campaign urges general practice to ensure that 100% of those eligible should receive the offer of a vaccination.3 To boost uptake in your at-risk groups, no opportunity should be missed to reinforce the importance of vaccination – whether that is at routine annual reviews, or opportunistically during any consultation.
This year, flu vaccinations will start in October, rather than the traditional September start to the campaign.3 This is because of evidence that the effectiveness of the vaccine can wane over time in adults. However, despite the delayed start, there is an expectation that the majority of vaccinations will be completed by the end of November, closer to the time that the flu season commonly starts. Vaccinating adults closer to the time when the flu virus is likely to circulate (which typically peaks in December or January) will provide optimal protection during the highest risk period.
TIMING
As flu circulation in children normally starts earlier than in adults, the Joint Committee on Vaccination and Immunisation (JCVI) has advised that the children’s programme should start in September, as soon as vaccine stocks are available.
Protection from the vaccine lasts much longer in children, so the priority is to start vaccinating all children (including those in clinical risk groups) as soon as possible, both to provide early protection to children and reduce transmission to the wider population.3
Nor should there be any change to the timing of the offer of vaccination to pregnant women. There are three clinical reasons to vaccinate pregnant women against flu:
- To protect the pregnant women themselves – they are at higher risk from complications from flu
- To protect the baby during pregnancy – flu increases the risks of the baby being born prematurely, or stillborn
- To protect the baby in the first few months of life (babies aged under 6 months are at high risk of complications from flu).
Although the first and second of these reasons would align with the advice to provide maximum protection during the flu season itself, the third reason requires women to be vaccinated prior to delivery, and therefore vaccination of pregnant women should begin from 1 September. Pregnant women are not expected to lose protection as rapidly as the elderly population and therefore starting vaccination (particularly in those women who are in the later stages of pregnancy) earlier than for those in other clinical risk groups, will still offer protection to women themselves in the peak season. Commencing vaccination early will, however, ensure that as many newborn babies as possible are protected during the flu season and help to optimise uptake.
Following clinical assessment, there may be a small number of other adults for whom it would be better not to delay flu vaccination until October. For example, for those who are due to start immunosuppressive treatment, e.g. chemotherapy, receiving the vaccine before they start treatment would allow them to make a better response to their vaccination. General practice nurses should use their clinical judgement to bring forward vaccination in exceptional circumstances, as outlined in the Green Book, and offer vaccination as soon as vaccine comes available.
From 1 September 2024
- Pregnant women
- All children aged 2 or 3 years on 31 august 2024
- Primary school aged children (from reception to year 6)
- Secondary school aged children (from year 7 to year 11)
- All children in clinical risk groups aged from 6 months to less than 18 years
From October 2024
- Those aged 65 years and over
- Those aged 18 years to under 65 years in clinical risk groups (as defined by The Green Book, influenza chapter 19)
- Those in long-stay residential care homes
- Carers in receipt of carer’s allowance, or those who are the main carer of an elderly or disabled person
- Close contacts of immunocompromised individuals
- Frontline workers in a social care setting without an employer-led occupational health scheme including those working for a registered residential care or nursing home, registered domiciliary care providers, voluntary managed hospice providers and those that are employed by those who receive direct payments (personal budgets) or personal health budgets, such as personal assistants
Practices are expected to deliver a 100% offer to eligible groups. They should ensure they make firm plans to equal or improve uptake rates in 2024 to 2025 (see Table 1), particularly in those groups where uptake has traditionally been lower (clinical risk groups, children aged 2 and 3 years, and pregnant women). Practices should also ensure they have robust plans in place for tackling health inequalities for all underserved groups.
THE VACCINES
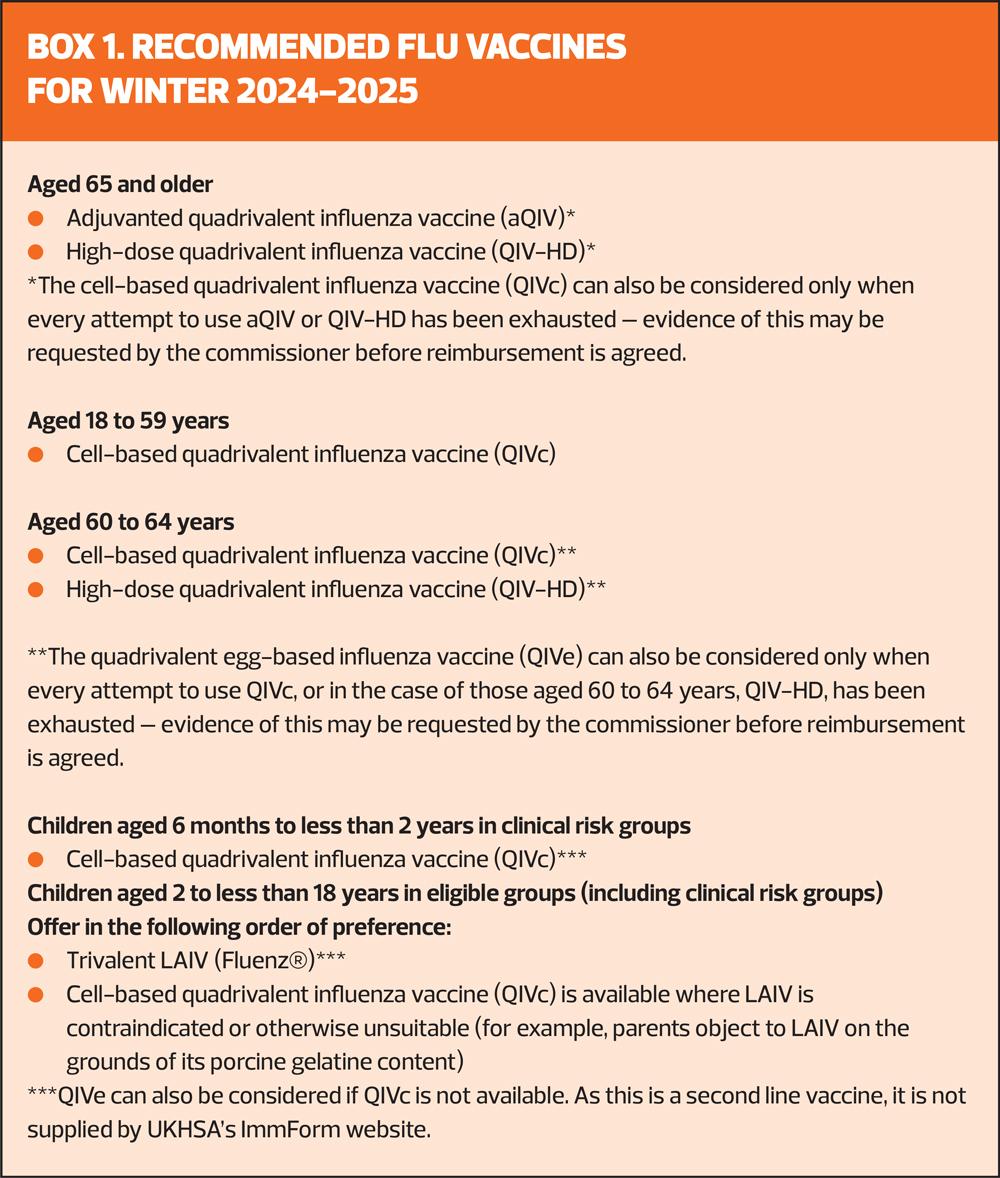
Recently, the vaccine manufacturer Sanofi announced that the recombinant quadrivalent influenza vaccine (QIVr) will not be available for this year’s flu vaccination programme.4 The recommended vaccines for this campaign are listed in Box 1.
All the vaccines for adults currently used in the UK programme are quadrivalent, containing four strains: two subtypes of influenza A and both lineages of influenza B. Since March 2020, however, there have been no confirmed detections of wild type B/Yamagata lineage by the UK national influenza centre or internationally. The public health risk from B/Yamagata is now considerably lower than at the time of the introduction of quadrivalent vaccines and B/Yamagata may be extinct. Therefore, trivalent influenza vaccines, without a B/Yamagata strain, are currently considered clinically suitable. As manufacturers start to produce trivalent products, their use will be preferred over the equivalent quadrivalent formulation.5 The live attenuated influenza vaccine (LAIV) for children will, this year, be a trivalent vaccine, with the brand name Fluenz® .
WHO6 RECOMMENDATIONS FOR 2024-25
Cell culture- or recombinant-based vaccines
- An A/Wisconsin/67/2022 (H1N1)pdm09-like virus;
- An A/Massachusetts/18/2022 (H3N2)-like virus; and
- A B/Austria/1359417/2021 (B/Victoria lineage)-like virus.
While the B/Yamagata lineage vaccine component of quadrivalent vaccines is no longer warranted, where quadrivalent vaccines are still in use for use in the 2024-2025 in the northern hemisphere, they should contain a B/Phuket/3073/2013 (B/Yamagata lineage)-like virus, unchanged from the previous year.
Trivalent, egg-based vaccines should contain:
- An A/Victoria/4897/2022 (H1N1)pdm09-like virus;
- An A/Thailand/8/2022 (H3N2)-like virus; and
- A B/Austria/1359417/2021 (B/Victoria lineage)-like virus.
Mismatches between the components in the vaccine and circulating viruses do occur from time to time and explain much of the variation in estimates of vaccine effectiveness. Even when antigenic drift occurs, vaccination is still recommended as some degree of protection may be provided against the drifted strain and the vaccine should still offer protection against the other strains in the vaccine. Historically, for the trivalent vaccines which contain an influenza B strain from a single lineage, mismatches between the vaccines and the circulating B strain occurred frequently. However, with the exception of 2014 to 2015, in most recent years the vaccines have closely matched the influenza A viruses circulating during the influenza season. After immunisation, protective immune responses may be achieved within 14 days.
OTHER VACCINATIONS
Practices are being encouraged to align delivery of the flu vaccination programme with other commissioned vaccination programmes for which the patient may be eligible (for instance, shingles, pertussis, or pneumococcal vaccines) where it is clinically acceptable, operationally feasible, and where the patient consents.3 Where co-administration may not be feasible, practices must make every effort to encourage individuals to take up the offer of every vaccine they are eligible for.
COVID-19
The Government published advice from the Joint Committee on Vaccination and Immunisation (JCVI) on the COVID-19 vaccine programme in autumn this year.7 Similar to previous spring and autumn campaigns, the JCVI’s advice is to offer the vaccine to those at high risk of serious disease and who are therefore most likely to benefit from vaccination.
JCVI advises the following groups should be offered a COVID-19 vaccine this autumn:
- Adults aged 65 years and over
- Residents in a care home for older adults
- Individuals aged 6 months to 64 years in a clinical risk group. Pregnant women are included in the clinical risk group.8
The primary aim of the national COVID-19 vaccination programme remains the prevention of severe illness (hospitalisations and deaths) arising from COVID-19. As currently available COVID-19 vaccines provide limited protection against mild and asymptomatic disease, the focus of the programme is on offering vaccination to those most likely to directly benefit from vaccination, particularly those with underlying health conditions that increase their risk of hospitalisation following infection.
The vaccine should usually be offered no earlier than around 6 months after the last vaccine dose, although there is scope for some flexibility as long as there is a minimum interval of 3 months between doses.
COVID-19 vaccination is not recommended for frontline health and social care workers, staff working in care homes for older adults, unpaid carers and household contacts of people with immunosuppression.
Infection with SARS-CoV-2 continues to occur throughout the year. The current trend suggests intermittent waves occurring every few months, but the peak rate of infection appears to be declining over time.7 Winter remains the period of greatest threat from COVID-19 both in relation to the risk of infection to individuals and the pressures on health systems.
Dr Mary Ramsay, Director of Public Health Programmes at the UK Health Security Agency, said: ‘Our on-going surveillance shows that COVID-19 continues to cause severe illness, putting many in hospital, particularly older people and those with underlying medical conditions. But it also shows that the autumn vaccines are effective in helping to give added protection to those most at risk – almost halving the likelihood of hospitalisation from the virus for a few months following vaccination and over the winter period.’
UKHSA surveillance data on last autumn’s programme showed that those who received a vaccine were around 45% less likely to be admitted to hospital with COVID-19 from two weeks following vaccination with protection lasting for around 4 months, compared with those who did not receive one.9
Vaccine uptake for last year’s autumn programme for those aged 65 years and over was approximately 70%.9
RSV
From 1 September 2024, two new respiratory syncytial virus (RSV) vaccination programmes have been introduced, as recommended by the JCVI. They are:
- A programme for older adults aged 75 to 79 years old
- A programme for pregnant women to protect infants
Despite infecting around 90% of children within the first 2 years of life, RSV is relatively unknown among the public. It typically causes mild, cold-like symptoms. However, it can lead to severe lung infections such as pneumonia and infant bronchiolitis and is a leading cause of infant mortality globally.
Each year in the UK, RSV accounts for around 30,000 hospitalisations in children aged under 5 and is responsible for 20 to 30 infant deaths. It also causes around 9,000 hospital admissions in those aged over 75. The RSV programme could free up thousands of hospital bed days and help to avoid hundreds of deaths each year.
The UK position differs from that in the US, where the US Centers for Disease Control and Prevention (CDC) recommends that everyone aged 75 or over, and people aged 60-74 at increased risk of serious RSV such as lung or heart disease, should receive the vaccine.10
CONCLUSION
It looks as if general practice nurses are in for a very busy time over the next month or two: despite starting the seasonal flu campaign later than usual, there is an expectation that everyone who is eligible should have received their vaccination by the end of November. Not only that, but this year there are not one but two additional vaccines to offer, at least to some patient groups. Nonetheless, flu vaccination is an integral part of winter planning for the NHS. Not only does it help to keep our patients out of hospital, but by vaccinating front line practice staff, it helps to reduce sickness absence – and with so much to do, the last thing anyone wants is for colleagues to go off sick.
REFERENCES
1. World Health Organization. Influenza (seasonal); October 2023. https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
2. UK Health and Security Agency. Seasonal flu vaccine uptake in GP patients, Winter season 2023 to 2024 https://www.gov.uk/government/statistics/seasonal-influenza-vaccine-uptake-in-gp-patients-winter-season-2023-to-2024/seasonal-influenza-vaccine-uptake-in-gp-patients-in-england-winter-season-2023-to-2024
3. Department of Health and Social Care. National flu immunisation programme 2024 to 2025 letter. https://www.gov.uk/government/publications/national-flu-immunisation-programme-plan-2024-to-2025/national-flu-immunisation-programme-2024-to-2025-letter
4. Department of Health and Social Care. Statement of amendment to the national flu immunisation programme 2024 to 2025 letter; June 2024. https://www.gov.uk/government/publications/national-flu-immunisation-programme-plan-2024-to-2025/statement-of-amendment-to-the-annual-flu-letter-for-2024-to-2025-12-june-2024
5. UKHSA. Immunisation against infectious disease: The Green Book – Chapter 19. Influenza; November 2023. https://assets.publishing.service.gov.uk/media/654cf306014cc90010677371/Green-book-chapter-19-influenza-_3November2023.pdf
6. World Health Organization. Recommended composition of influenza virus vaccines for use in the 2024-2025 influenza season in the Northern Hemisphere; February 2024. https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2024-2025-northern-hemisphere-influenza-season
7. Joint Committee on Vaccination and Immunisation. JCVI statement on the COVID-19 vaccination programme for Autumn 2024. https://www.gov.uk/government/publications/covid-19-autumn-2024-vaccination-programme-jcvi-advice-8-april-2024/jcvi-statement-on-the-covid-19-vaccination-programme-for-autumn-2024-8-april-2024
8. UKHSA. Immunisation against infectious disease: The Green Book – Chapter 14a. COVID-19; updated April 2024. https://www.gov.uk/government/publications/covid-19-the-green-book-chapter-14a
9. UKHSA. JCVI advises on eligible groups for autumn COVID-19 vaccination. (Press release); August 2024. https://www.gov.uk/government/news/jcvi-advises-on-eligible-groups-for-autumn-covid-19-vaccination
10. Centers for Disease Prevention. CDC updates RSV vaccination recommendation for adults (press release); June 2024. https://www.cdc.gov/media/releases/2024/s-0626-vaccination-adults.html
Related articles
View all Articles