Masterclass: Childhood immunisation
Dr Mary Lowth
Dr Mary Lowth
MA MB BChir FRCGP PGCMedE
GP and MRCGP examiner
Childhood immunisation is a three-legged stool: the three legs of knowledge, consultation skills and practical technique are all needed in order to deliver this service effectively and well. An article like this cannot substitute for practical teaching and experience in immunisation technique, neither does it cover communication skills for dealing with children who are to be immunised. It aims therefore to give some facts with which to underpin the other two essential skills.
WHAT IS IMMUNISATION?
When we talk about childhood immunisation we are describing a process of ACTIVE immunisation – that is of stimulating the immune system of the child to a point at which it can mount its own defence if it ever encounters the disease in question.
This differs from passive immunity, in which the recipient is given immunoglobulin against a particular disease. Babies are passively immunised by their mothers, through the passage of maternal immunoglobulin across the placenta. The effect is very protective to the newborn but has little effect beyond 6 months of age as the antibodies are eventually lost and the baby does not have their own natural immune response to replace the antibodies. Immunisation aims to replace this, for the important and dangerous diseases of childhood.
HISTORY OF IMMUNISATION
Edward Jenner, the father of immunisation, was a doctor from Gloucestershire. He investigated the country ‘lore’ that that cowpox, a harmless infectious disease of milkmaids, gave protection against smallpox. He famously inoculated a local boy with cowpox and then proved him to be immune to smallpox. His work began our understanding of immunity and thus our ability to protect against serious infectious disease.
Smallpox, once one of the world’s greatest killer diseases, was officially declared eradicated from the whole world in 1979.
Formal widespread vaccination programmes began in the twentieth century. In the UK, diphtheria immunisation began in 1940, pertussis in the 1950s, BCG in 1953, polio in 1958, tetanus in 1961, measles in 1968, rubella in 1970, MMR in 1988, meningitis C in 1999 and pneumococcus in 2006. In 2008 human papillomavirus (HPV) vaccination was introduced into the routine childhood immunisation schedule. Rotavirus and shingles vaccination were added to the programme in 2013.
INDIVIDUAL AND COMMUNITY BENEFITS
Vaccines teach the immune system by mimicking a natural infection. The mock infection is cleared, and humans are left primed to fight off a future attack by the organism before it can take hold and cause disease.
The benefits of vaccination go beyond avoiding the disease directly. If your immune system stops an illness before it starts, you will not pass that disease on. If a critical number of people within a community are vaccinated against a particular illness, the entire group becomes less likely to get the disease. This protection is called herd immunity.
If too many people in a community refuse vaccinations, as we have seen with the recent falls in MMR uptake, diseases can reappear. In 1989 a measles outbreak in the United States resulted in more than 55,000 cases of measles and 136 measles-associated deaths.
TYPES OF VACCINE
For the practice nurse the most important consideration is generally whether or not the vaccine is live. However there are a number of different vaccines types in addition to live vaccines, and it’s useful to understand these. Remember that all offer ACTIVE rather than passive immunisation.
Live, attenuated vaccines
Live, attenuated vaccines contain a version of the living microbe that has been weakened so it can’t cause disease. These vaccines are good “teachers” of the immune system because they closely simulate true infection, so they may confer lifelong immunity with only one or two doses.
The downside is that there is a remote possibility that attenuated microbe can mutate back to a dangerous form and cause disease. This happened with live polio vaccine in the 1950s, and as a result we use an inactivated vaccine against polio these days.
Not everyone can be given live, attenuated vaccines. Immunosuppressed patients are at risk of overwhelming infection if given them. This includes patients on chemotherapy, who need protection against infectious disease. Additionally such vaccines normally need refrigeration within a set temperature range, to keep the organisms alive but stop them multiplying too rapidly.
Viral vaccines are particularly easy to make live, as they are simple genetic structures and easier to weaken in the lab: measles, mumps, and chickenpox, for example, are live vaccines. Bacteria are more complex organisms and harder to control and render harmless, so very few bacterial vaccines are live.
Inactivated Vaccines
Inactivated vaccines are made by first killing the microbe, leading to a stable, safe, dead vaccine which can’t mutate and doesn’t need to be kept cold. It may even be freeze-dried (hence it may be useful in developing countries’ where refrigeration is impractical).
Inactivated vaccines tend to stimulate a weaker response than do live vaccines, perhaps because it’s not quite so ‘real’, and so boosters are more likely to be needed over time. Polio is an inactivated vaccine.
Subunit Vaccines
Subunit vaccines include only the part of the microbe that stimulates the immune system. This means that they have most of the advantages of inactivated vaccines, but the chances of adverse reactions are lower.
A subunit vaccine has been made for hepatitis B, and research is on going on a subunit vaccine against hepatitis C.
Toxoid Vaccines
For bacteria such as tetanus that cause illness through secretion of toxins, a toxoid vaccine can be the answer. Toxins are inactivated with formalin, and are then rendered harmless and safe for use in vaccines, and the immune system learns how to fight off not the bacteria but the toxin.
Conjugate Vaccines
Some bacteria wear a ‘false coat’ of sugars in order to block themselves from immune attack. Conjugate vaccines ‘label’ these polysaccharides with proteins from a microbe that an infant’s immune system DOES recognise – rather like painting a target on them. The linkage helps the immune system react and defend against the sugarcoated bacterium. The vaccine that protects against Haemophilus influenzae type B (Hib) is a conjugate vaccine.
DNA Vaccines
Still in the experimental stages, DNA vaccines dispense with the organism and get right down to its genetic material. In particular, DNA vaccines use the genes that code for the surface antigens of the microbe. These vaccines are taken up by the body’s own DNA so the body starts to makes the microbial antigen, and then produces antibodies to it. DNA vaccines being tested include vaccines against herpes.
Recombinant Vector Vaccines
Recombinant vector vaccines are similar to DNA vaccines, but they use an attenuated virus or bacterium to introduce microbial DNA to cells. Researchers are working on this type of vaccine for HIV and rabies.
Adjuvants
Adjuvants are substances added to the vaccine to enhance the immune response to it. This can mean needing a reduced dosage of active component for the same effect, reducing risks of allergy and side effects. It can also reduce cost.
ROUTES OF IMMUNISATION
Most vaccines are given intramuscularly, but subcutaneous, intradermal and oral administration routes are still important for some vaccines.
The benefit of intramuscular injection is that local reactions are minimised and immune response enhanced by depositing vaccine into the muscle. Vaccine needs to be injected slowly to avoid pain and muscle trauma.
Some vaccines (e.g. inactivated polio, varicella and meningococcal polysaccharide vaccines) are only licensed for subcutaneous administration.
For subcutaneous injection, the needle is held at a 45° angle to the skin. The standard needle for SC injection is a 25 or 26 gauge, 16 mm long.
For intradermal injection a 26 or 27 gauge, 10 mm needle is recommended. The intradermal injection technique requires special training from a trained provider.
The choice of injection sites depends primarily on the age of the person to be vaccinated. The two anatomical sites recommended as routine injection sites are the anterolateral thigh and the deltoid muscle.
It is important that infants and children do not move during injection of vaccines. However, excessive restraint can increase their fear and result in increased muscle tension. With practical training and experience you will be able to use a variety of positions for vaccinating different age groups without excessive force or discomfort.
Multiple vaccines
When sequentially administering multiple vaccines to children, give the most painful vaccine last (e.g. pneumococcal conjugate vaccine). Evidence suggests that this may decrease the overall pain response.
The location of each separate injection given should be recorded, so that if a local adverse event occurs, the implicated vaccine(s) can be identified.
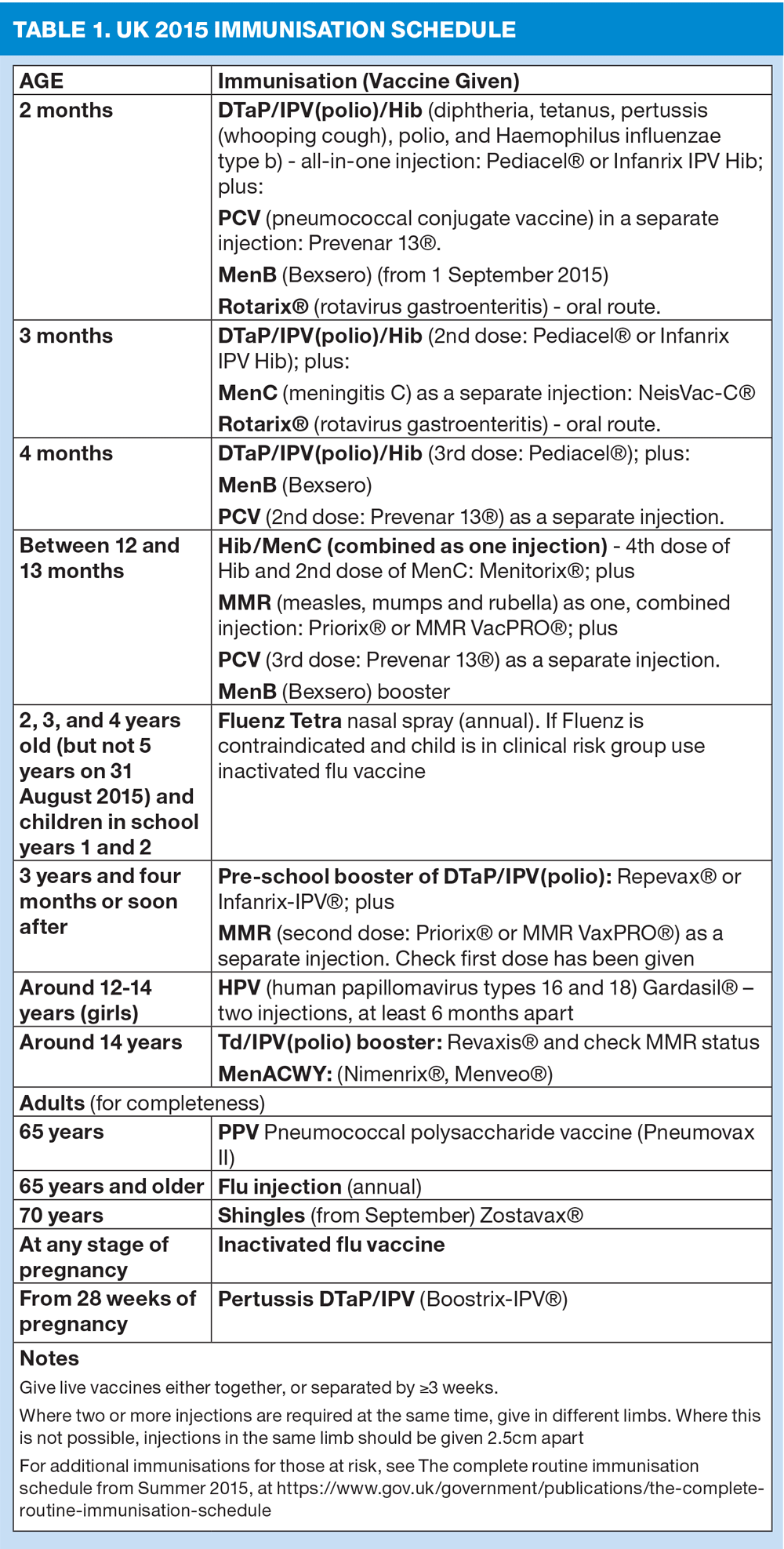
What are the routine immunisations?
See Table 1. Current recommendations can be found in the Green Book, at https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/400554/2902222_Green_Book_Chapter_11_v2_4.pdf
A note on chicken pox vaccine
The introduction of a mass chickenpox vaccination programme in the UK has been delayed due to concerns that an increase in shingles would occur. This has been seen in the USA since the introduction of mass vaccination there.
A vaccine is available in the UK and is offered to healthcare workers who may come into contact with the disease while not immune.
Public Health England also states that the vaccine may be given to children aged 1-12 years who are close contacts of people considered to be at high risk of severe chickenpox or shingles infection. It is also licensed for healthy adults and children over 13 years old who are not immune to chickenpox (indicated by blood tests). This gives practice nurses considerable leeway to give the vaccine to patients of all ages.
Two doses of vaccine are given 4-8 weeks apart. The two-dose schedule affords 98% protection in children and 75% protection in adolescents. Those who have had the disease are usually immune but second and even third attacks are reported, especially if the first was mild.
CONTRAINDICATIONS
1. Allergies
It is important to assess for a history of allergies and previous reactions to vaccines prior to giving any dose of vaccine. Vaccines rarely produce allergy or anaphylaxis. Overall, the risk of anaphylaxis after a single vaccine has been estimated as less than 1 case per 1 million.
Anyone who experiences an allergic reaction associated with a vaccine dose needs to be investigated to ascertain whether vaccination was the cause, and determine under what circumstances further vaccination can be provided. Where there is any doubt, rather than withholding vaccine, advice should be sought from secondary care.
All vaccines are contra-indicated in primary care in those who have had a confirmed anaphylactic reaction to a previous dose of a vaccine containing the same antigens or to its components.
Individuals with a confirmed anaphylactic reaction to egg should not receive influenza, rabies or yellow fever vaccines in primary care, and specialist advice should be sought. A number of studies have shown that current very low egg albumin levels in influenza vaccines are in fact safe, but immunisation in such cases should be in secondary care.
Generally other vaccines are safe, including MMR, which contains only a negligible quantity of egg ovalbumin. MMR vaccination is NOT contraindicated in children with egg allergy (even anaphylaxis).
2. Immunosuppression
Live vaccines may be temporarily or permanently contra-indicated in individuals who are:
- Immunosuppressed (transplants, chemotherapy, HIV)
- Pregnant
- Particularly unwell (e.g. pyrexia, URTI)
The following are NOT contraindications:
- Family history of any adverse reactions following immunisation
- Previous history of pertussis, measles, rubella or mumps infection
- Prematurity: immunisation should not be postponed
- Stable neurological conditions such as cerebral palsy and Down’s syndrome
- Contact with an infectious disease
- Asthma, eczema, hay fever or ‘snuffles’
- Treatment with antibiotics or locally-acting (e.g. topical or inhaled) steroids
- Child’s mother is pregnant
- Child being breastfed
- History of jaundice after birth
- Under a certain weight
- Over the age recommended in immunisation schedule
- Treated with ‘replacement’ corticosteroids
CONTACT WITH PREGNANT WOMEN
It is safe to administer live vaccines to the children of pregnant women. There is no risk of transmission of measles, mumps or rubella vaccine viruses. There is an almost negligible risk of transmission of varicella-zoster vaccine virus: vaccine recipients who develop a varicella-like rash should be advised to cover the rash if in contact with a pregnant woman. There is a very small possibility of transmission of the rotavirus vaccine but the benefit of immunising infants far outweighs any theoretical risk.
PRETERM INFANTS
Prematurity, particularly extreme prematurity (<28 weeks gestational age) can place children at increased risk of vaccine-preventable disease. Despite their immunological immaturity, preterm infants generally respond satisfactorily to vaccines, and should usually be vaccinated according to the recommended schedule at the usual chronological age, without correction for prematurity.
IMMUNOCOMPROMISED PERSONS
Vaccination presents several challenges in immunocompromise:
- Immune protection from previous vaccinations may be reduced
- The response to vaccines may be reduced
- The risk of vaccine-preventable diseases may be increased
- The risk of adverse events from live vaccines may be increased
Degrees of immunocompromise vary from insignificant to profound, and may be temporary (e.g. chemotherapy). This, together with the risk of acquiring vaccine-preventable disease, should be taken into account when considering a vaccination schedule.
In adults, daily doses of oral corticosteroids in excess of 60 mg of prednisolone for more than 1 week cause significant immunocompromise.
In children, doses in excess of either 2 mg/kg per day for over 1 week or 1 mg/kg per day for over 4 weeks cause significant immunocompromise.
Lower doses of steroids cause some impairment of the immune response.
Live attenuated vaccines are generally contraindicated until at least 4 weeks post cessation of treatment.
RECENT IMMIGRANTS
Refugees or immigrants may be incompletely vaccinated or have incomplete records.
If there is a valid record of vaccination from overseas, the history of previous doses should be taken into account when planning a catch-up vaccination schedule. However, some doses may be invalid, as the interval between doses may be too short. If a migrant/refugee has no valid documentation of vaccination, a standard ‘catch-up’ schedule should be commenced.
CONSENT TO VACCINATION
Vaccination is an invasive procedure, and informed consent is required. Minimum information would be for the patient or guardian to know what the vaccine was for, what disease it is to prevent, the risks and side effects from the vaccine and the risks of the disease if the vaccine is refused.
Consent may be written, verbal or implied (e.g. by rolling up your sleeve willingly) and must be obtained before each injection.
In children under 16 years of age, consent should be obtained from an individual with ‘parental responsibility’. Where this person brings the child in response to an invitation for immunisation, and following an appropriate consultation, presents the child for that immunisation, these actions may be considered as evidence of consent.
A child aged under 16 may consent or refuse, providing they are Gillick competent. Ideally their parents will be involved. If they refuse then it is unlikely that a person with parental responsibility could overrule such a refusal, although it is possible that the court might do so.
Summary
Vaccination is a large part of the practice nurse’s remit, and it’s a huge area in which the opportunity to become expert can be seen as intimidating or exciting. We hope you will see it as exciting. A number of excellent websites support further more detailed knowledge in all the areas discussed above, and the current UK immunisation schedule is given in Box 1.
Related articles
View all Articles