NICE NG28: Major update to type 2 diabetes in adults guideline
DR GERRY MORROW
DR GERRY MORROW
MB ChB MRCGP Dip CBT
Editor CKS, Medical & Product Director (Primary Care) Agilio Software
Practice Nurse February 2022;52(2): online only
In February 2022, NICE published a significant update to its management guideline for type 2 diabetes. This included new recommendations in three areas, first-line drug treatment, reviewing drug treatments, and treatment options if further interventions are needed. Some of these have far reaching consequences for people with type 2 diabetes and for general practice nurses who are involved in the care and management of these patients.
Taking each of these areas of management in turn, the most significant change is in the first-line drug treatment options.
FIRST-LINE DRUG TREATMENT
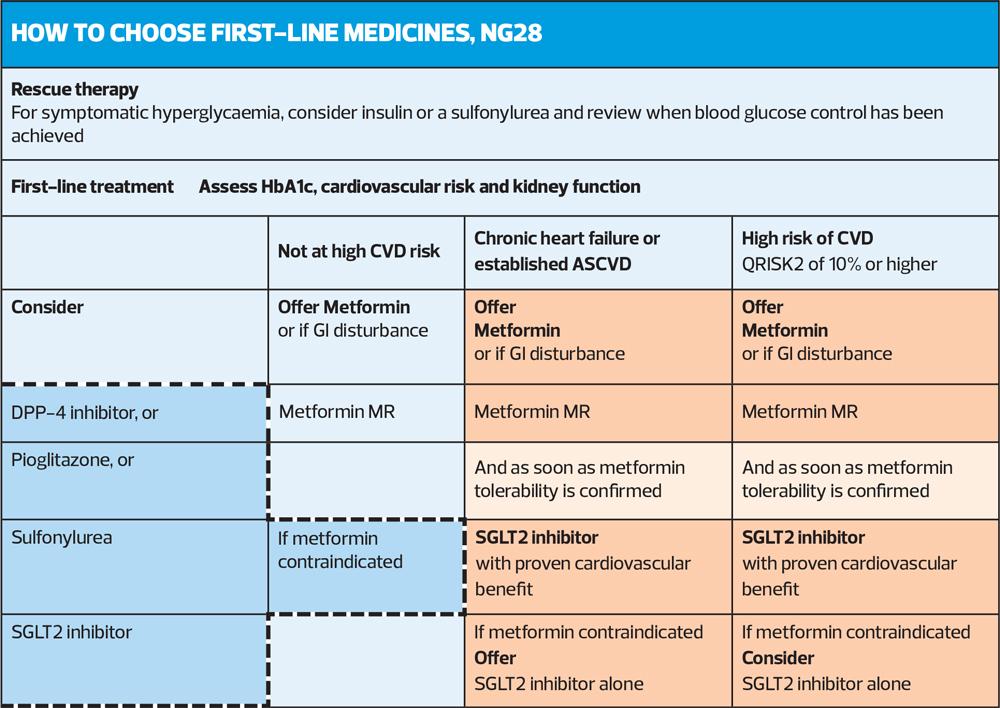
Where previously metformin was the recommended monotherapy, now the advice from NICE is that all patients should have a cardiovascular risk status assessment to determine the therapeutic interventions at first-line.
People identified as having chronic heart failure, established atherosclerotic cardiovascular disease (ASCVD) or at high risk of developing cardiovascular disease (defined as a QRISK2 score of more than 10% in adults aged 40 and over or an elevated lifetime risk of cardiovascular disease [defined as the presence of one or more cardiovascular risk factors in someone under 40]) should be prescribed metformin and a SGLT2 inhibitor.
The drugs should be started sequentially, starting with metformin. The SGLT2 inhibitor should be started once the person is has achieved tolerability of metformin at their optimal dose. There are also caveats outlined about issues to consider before someone commences an SGLT2 inhibitor, given the increased risk of developing diabetic ketoacidosis (DKA) with these medications. Specific areas to consider are whether the individual has had any episodes of DKA previously, if they are currently unwell, or the person is following a very low carbohydrate or ketogenic diet.
For those not defined as at cardiovascular risk metformin monotherapy is still the recommended first-line treatment.
For people who cannot tolerate metformin, or they have a contraindication, NICE states that the options to consider are either:
- A DPP-4 inhibitor, or
- Pioglitazone, or
- A sulfonylurea, or
- An SLGT2 inhibitor (only if a DPP"‘4 inhibitor would otherwise be prescribed, and a sulfonylurea or pioglitazone is not appropriate).
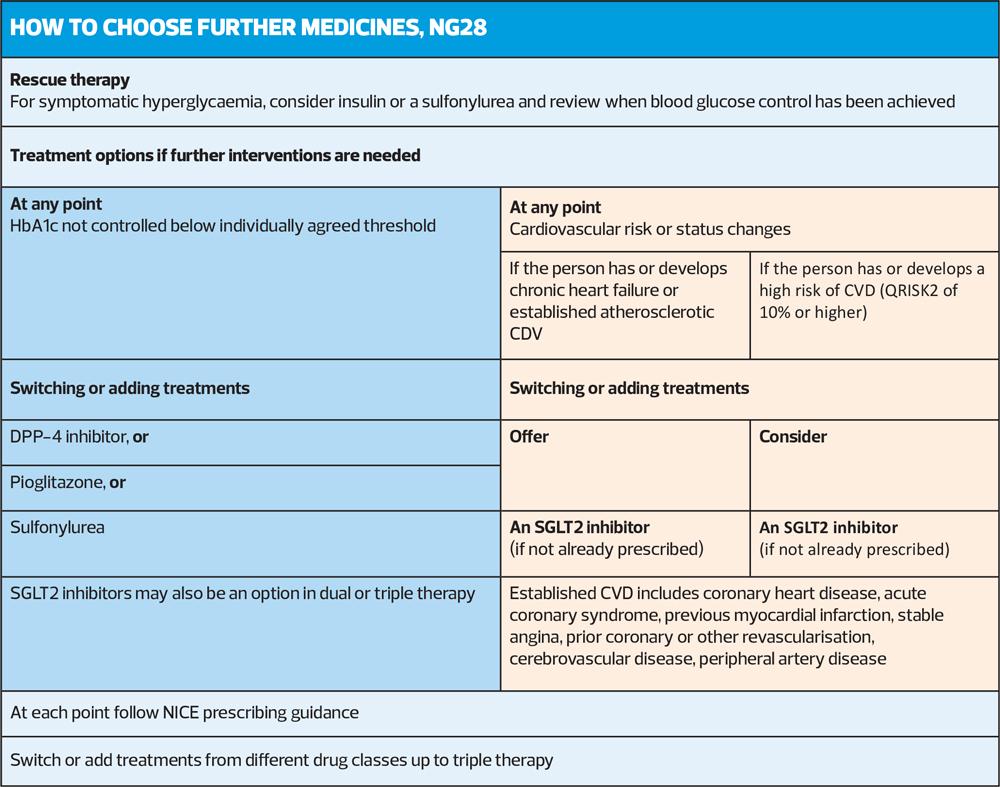
REVIEWING DRUG TREATMENTS
When practice nurses are and reviewing the response to therapy of people with type 2 diabetes, the updated NICE guideline again recommends the introduction of SGLT2 inhibitors if the person develops cardiovascular disease or risk factors, which are hypertension, dyslipidaemia, smoking, obesity, and family history of premature cardiovascular disease (in a first-degree relative).
The NICE guideline committee (GDC) advises that you should consider adding an SGLT2 inhibitor to the current treatment or, instead, replace an existing drug with an SGLT2 inhibitor.
The GDC also advises that prescribers take into account the person's current treatment regimen and preferences and make a shared decision about switching treatments or adding an SGLT2 inhibitor, as appropriate.
This shared decision-making approach is widely advised across NICE guidelines and has proven benefits for patients and clinicians alike. It may, however, be a challenge when the treatment regimen means increasing numbers of medications for patients who may already be burdened by polypharmacy. The time to explain in detail the pros and cons and the potential need for longer appointments and repeated reviews to consider the options can also be problematic for practice nurses under pressure to deliver more reviews for more people in the current pandemic world.
Treatment options if further interventions are needed
This final section finds a repeat of earlier advice to introduce SGLT2 inhibitors for people with cardiovascular disease or identified risks. However, as you would expect it also describes the stepwise decision-making process of which drugs to consider of dual therapy of metformin and another oral drug has not controlled HbA1c to the individual’s agree treatment threshold.
In this situation the advice is to move to triple therapy by adding one of the following; a DPP"‘4 inhibitor, pioglitazone, or a sulfonylurea, or an SGLT2 inhibitor, or to start insulin.
For those people where metformin is contraindicated or not tolerated and dual therapy with two oral drugs has not been effective in achieving appropriate control, the advice is to consider insulin-based treatment.
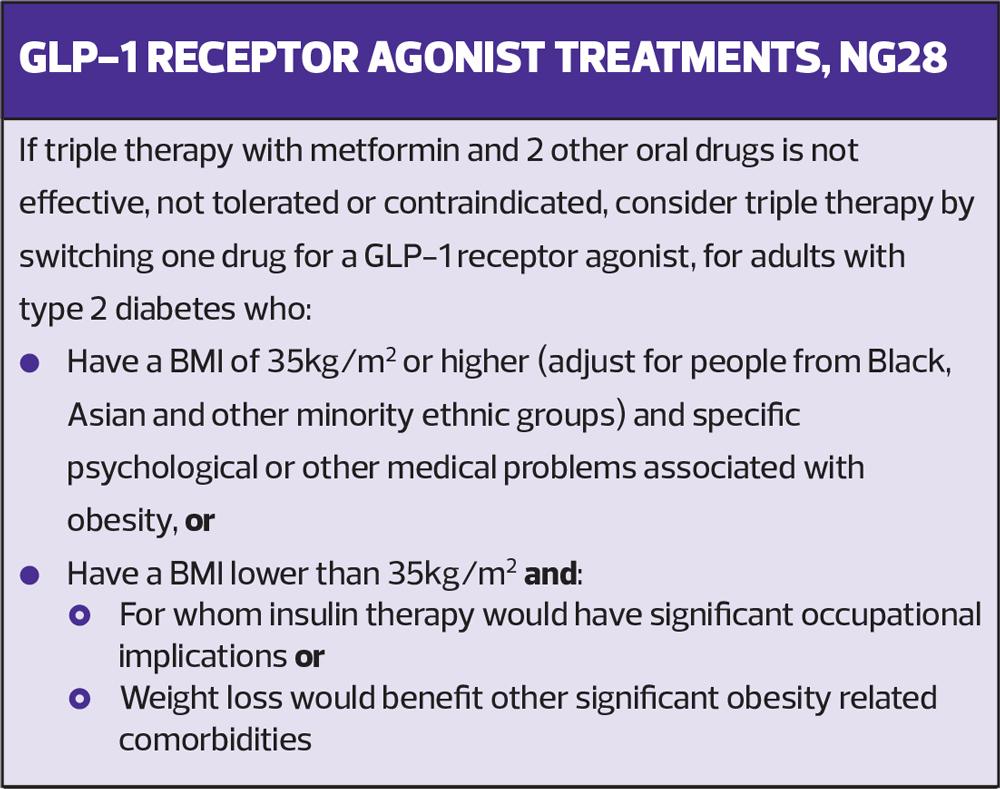
If this triple therapy with metformin and two other oral drugs is not effective, not tolerated or contraindicated, then the advice is to consider a different triple therapy by switching one drug for a GLP"‘1 receptor agonist (RA) in people who either have a BMI of 35 kg/m2 or higher and specific psychological or other medical problems associated with obesity or have a BMI lower than 35 kg/m2 and for whom insulin therapy would have significant occupational implications or weight loss would benefit other significant obesity-related comorbidities.
This final recommendation has proved to be somewhat controversial for some, particularly as the American Diabetes Association and European Association for the Study of Diabetes consensus report highlighted the benefits of GLP-1 RAs in reducing major adverse cardiovascular events, hospitalisation for heart failure, and chronic kidney disease progression.
It remains to be seen whether NICE revises its view as their detailed evidence review concluded that for major adverse cardiovascular outcomes, only injectable semaglutide showed clinically meaningful improvement when compared with DPP-4 inhibitors, sulfonylurea, and placebo. NICE also advised that, while oral semaglutide showed a clinically meaningful reduction in risk of all-cause mortality estimate against placebo, it had greater imprecision (broader 95% confidence intervals; HR 0.51, [0.31 to 0.84]) than was seen for empagliflozin versus placebo (HR 0.68, [0.57 to 0.82]).
Additionally, the NICE decision to advise that GLP-1 agonists be used fourth-line to treat type 2 diabetes was partly due to a cost-effectiveness consideration as they concluded that GLP-1 agonists as a class were not likely to be cost-effective in improving the cardiovascular prognosis of people with type 2 diabetes.
Further reading
NICE NG28. Type 2 diabetes in adults: management; updated 2022. https://www.nice.org.uk/guidance/ng28
NICE Type 2 diabetes [B] Pharmacological therapies with cardiovascular and other benefits in people with type 2 diabetes NICE guideline NG28. Evidence review underpinning recommendations 1.7.4-1.7.6 and 1.7.9-1.7.15 in the NICE guideline; February 2022. https://www.nice.org.uk/guidance/ng28/evidence/pharmacological-therapies-with-cardiovascular-and-other-benefits-in-people-with-type-2-diabetes-pdf-10956473392
Buse, J et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020;43(2):487-493
Related guidelines
View all Guidelines