Intrauterine Contraception: FSRH
This guidance from the Faculty of Sexual and Reproductive Healthcare provides an overview of intrauterine contraception methods, and their advantages over user-dependent methods
NB. This guidance has been updated: see FSRH Clinical guideline: intrauterine contraception; 2023 (updated 2025) https://www.fsrh.org/Public/Documents/ceu-guidance-intrauterine-contraception.aspx
In contrast to methods that depend on correct and consistent use by the woman, the effectiveness of long-acting reversible contractive (LARC) methods does not depend on adherence by the user.2 However, the uptake of LARC remains low (around 8% of contraceptive usage, according to NICE.2) The reasons for this include lack of training for healthcare professionals to equip them to help women make an informed choice from the full range of contraceptive methods.
This guideline summary does not attempt to cover all LARC methods, but to look at intrauterine contraception. Future 'Guidelines in a Nutshell' summaries will look at other methods, such as injectable contraceptives and implants.
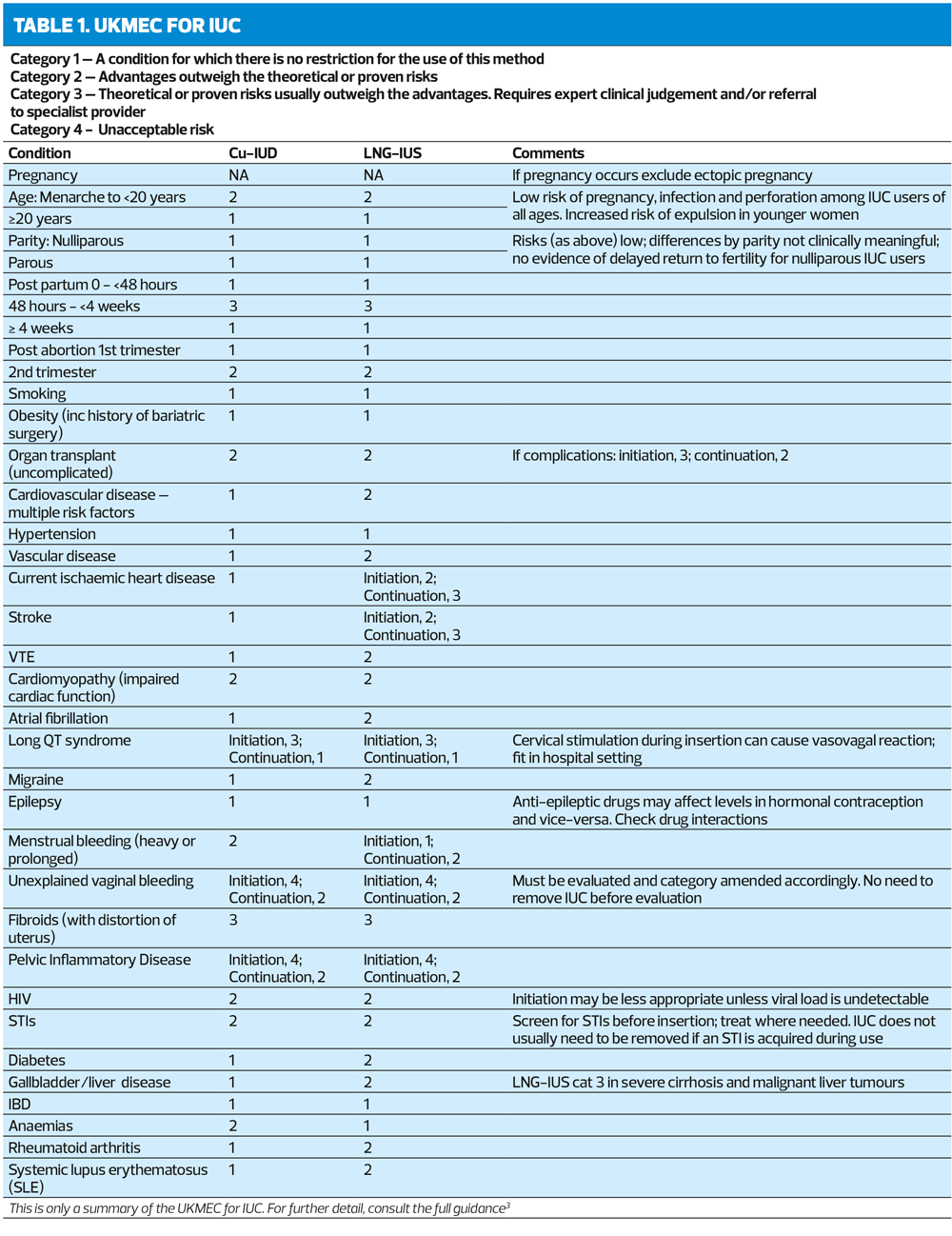
Intrauterine contraception (IUC) is highly effective and long-acting, ranging from 3 to 10 years.3 IUC comprises two types:
- Copper-bearing intrauterine device (Cu-IUD)
- Levonorgestrel-releasing intrauterine system (LNG-IUS).
Cu-IUDs have copper on their central stems and may also have copper on the arms. Those that contain the highest surface areas of copper are generally the most effective and are recommended first-line.3
Several LNG-IUS are now available, with two doses of LNG. The 13.5mg LNG-IUS (releasing 6μg LNG/day) are licensed for 3 years, and the 52mg LNG-IUS (releasing 20 μg LNG/day) for 5 years. UK Medical Eligibility Criteria (UKMEC) categories apply to both doses.3
FSRH RECOMMENDATIONS
- You should be familiar with the UKMEC for intrauterine methods. A summary is shown in Table 1
- Advise women of very low failure rates with IUC
The most effective methods are LNG-IUS and T-shaped Cu-IUDs with at least 380mm2 copper and copper bands on transverse arms
- Take medical and sexual history as part of routine assessment for suitability of use and need for STI screening
Health benefits and risks
- Cu-IUD use may be associated with reduced risk of endometrial and cervical cancers
- The 52mf LNG-IUS may reduce pain associated with primary dysmenorrhea, endometriosis or adenomyosis; may reduce menstrual loss and can be used in the management of heavy menstrual bleeding
- In the first 3-6 months of IUC use, women may experience irregular, prolonged or frequent bleeding but menstrual patterns usually settle with time
- Most women using the LNG-IUS experience infrequent bleeding at 1 year, and some will develop amenorrhea
- The overall risk of ectopic pregnancy is reduced with IUC use compared with using no contraception, but if pregnancy does occur, the risk of ectopic pregnancy is increased. Inform women of the symptoms of ectopic pregnancy, and consider ectopic pregnancy using IUC who present with abdominal pain and missed periods (or if amenorrhoeic, the woman starts bleeding). A positive pregnancy test should be followed with an ultrasound scan to locate the pregnancy
Complications
- The risk of expulsion with IUC is around 1 in 20, most commonly in the first year, particularly in the first 3 months
- The rate of uterine perforation with IUC is up to 2 per 1,000 insertions, and is higher in breastfeeding women
- Return to fertility is generally similar to fertility rates after discontinuation of oral contraception and barrier methods
- Cu-IUD users who experience recurrent bacterial vaginosis or candida may wish to change to an alternative method
Insertion
- Valid consent should be given by the woman before pelvic examination, and insertion or removal of IUC
- A bimanual pelvic examination should be performed on all women before inserting IUC
- Have an appropriately trained assistant present to monitor the woman and assist in the event of an emergency during insertion
- A Cu-IUD can be inserted at any time during the menstrual cycle if reasonably certain that the woman is not pregnant (unless required for EC). As it is effective immediately, no additional contraceptive precautions are required.
- The LNG-IUS can be inserted at any time during the menstrual cycle if reasonably certain that the woman is not pregnant, and up to day 7 in cycle without the need for additional contraception, or with additional contraception for 7 days if inserted later.
- For guidance on IUC insertion when switching from another contraceptive method, post partum or following termination of pregnancy, see full guideline.2
- There is no evidence that topical anaesthetics or NSAIDs reduce pain during insertion of IUC, but NSAIDs can be offered to women who experience pain after insertion
- There is no evidence that cleansing the cervix before IUC insertion reduces the risk of subsequent pelvic infection
Management of complications
- For LNG-IUS users with unscheduled bleeding, who wish to continue the method, and who are medically eligible, can be offered a combined oral contraceptive (in the usual cyclic manner or continuously without a pill-free interval [unlicensed use])
- Consider NSAIDs (or mefanimic acid or tranexamic acid) for problematic bleeding with Cu-IUDs
- IUC removal is not routinely required in women with pelvic inflammatory disease but the device should be removed if there is no response to treatment in 72 hours
- Women should be taught how to check for the IUC and warned that if the threads cannot be felt, the device may have perforated the uterus or been expelled: additional contraception should be used until they seek medical advice
- Women should be advised to seek medical advice at any time if:
– They develop symptoms of pelvic infection
– Pain
– Abnormal bleeding
– Late menstrual period (Cu-IUD)
– Non-palpable threads, or
– They can feel the stem of the IUC
Other issues
- Advise women of the need to use additional protection against STIs
- Inform women about the availability of emergency contraception and when it may be required with intrauterine methods i.e. if recent intercourse has occurred and the IUC is to be removed, for those who do not take additional precautions when indicated after an LNG-IUS is fitted, or if the IUC is used for longer than its licensed duration.
- Use of IUC should not be restricted on the basis of age or parity
- It's OK to use Mooncups or tampons – not associated with increased risk of expulsion
- For women with cardiac disease, the decision to use IUC should involve a cardiologist. IUC should be fitted in a hospital setting if vasovagal reaction poses high risk of cardiac event
References
1. NICE CG30. Long-acting reversible contraception (Full guideline), 2014. https://www.nice.org.uk/guidance/cg30/evidence
2. FSRH. Intrauterine contraception. https://www.fsrh.org/Public/Documents/ceu-guidance-intrauterine-contraception.aspx
3. FSRH. UKMEC 2016. http://www.fsrh.org/standards-and-guidance/external/ukmec-2016-digital-version/
Related guidelines
View all Guidelines