Guidelines in a Nutshell: GINA 2024 How to improve long-term outcomes for people with asthma
Mandy Galloway,
Mandy Galloway,
Editor
Practice Nurse 2024;54(3):8-11
The latest update to the Global Initiative for Asthma report, GINA 2024, published to coincide with World Asthma Day, is essential reading for any general practice nurse involved in, or responsible for, the management of patients with respiratory illness
Despite efforts to improve the management of asthma over the past 30 years, many patients have not benefited from advances in asthma treatment and still, even in the UK, do not receive the recognised basics of asthma care.
To improve asthma care and patient outcomes, evidence-based recommendations must also be disseminated and implemented, and integrated into clinical practice.
The safest and most effective approach to asthma treatment, and which avoids the consequences of starting treatment with short-acting beta2 agonist (SABA) alone and requires only a single medication, is to use a combination of inhaled corticosteroid and formoterol (ICS-formoterol) across all asthma severity levels.
There is also increasing concern about the impact of propellants in pressurised metered-dose inhalers (pMDIs) which contribute significantly to the carbon footprint of healthcare, particularly from use of SABAs.
The GINA Track 1 approach – which will be explained in more detail below – not only provides a large reduction in exacerbations, and in the risk of adverse effects of oral corticosteroids but also, if implemented with a dry powder inhaler (DPI) provides a large reduction in the carbon footprint of asthma care.
This summary focuses on the recommendations for adults and adolescents
DEFINITION
Asthma is a heterogenous disease, usually characterised by chronic airway inflammation, and defined by the history of respiratory symptoms such as wheeze, shortness of breath, chest tightness and cough, that vary over time. Airflow limitation may later become persistent.
Diagnosis
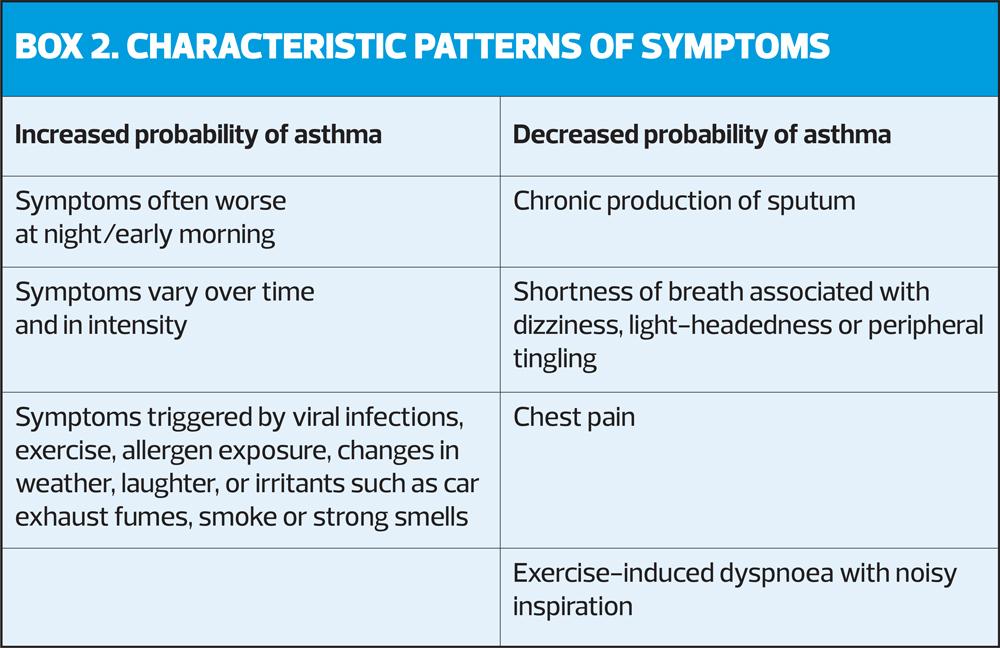
The diagnosis of asthma is based on the history of characteristic symptoms and evidence of variable expiratory airflow limitation. The pattern of symptoms is important, as respiratory symptoms may be due to acute or chronic conditions other than asthma (Box 2).
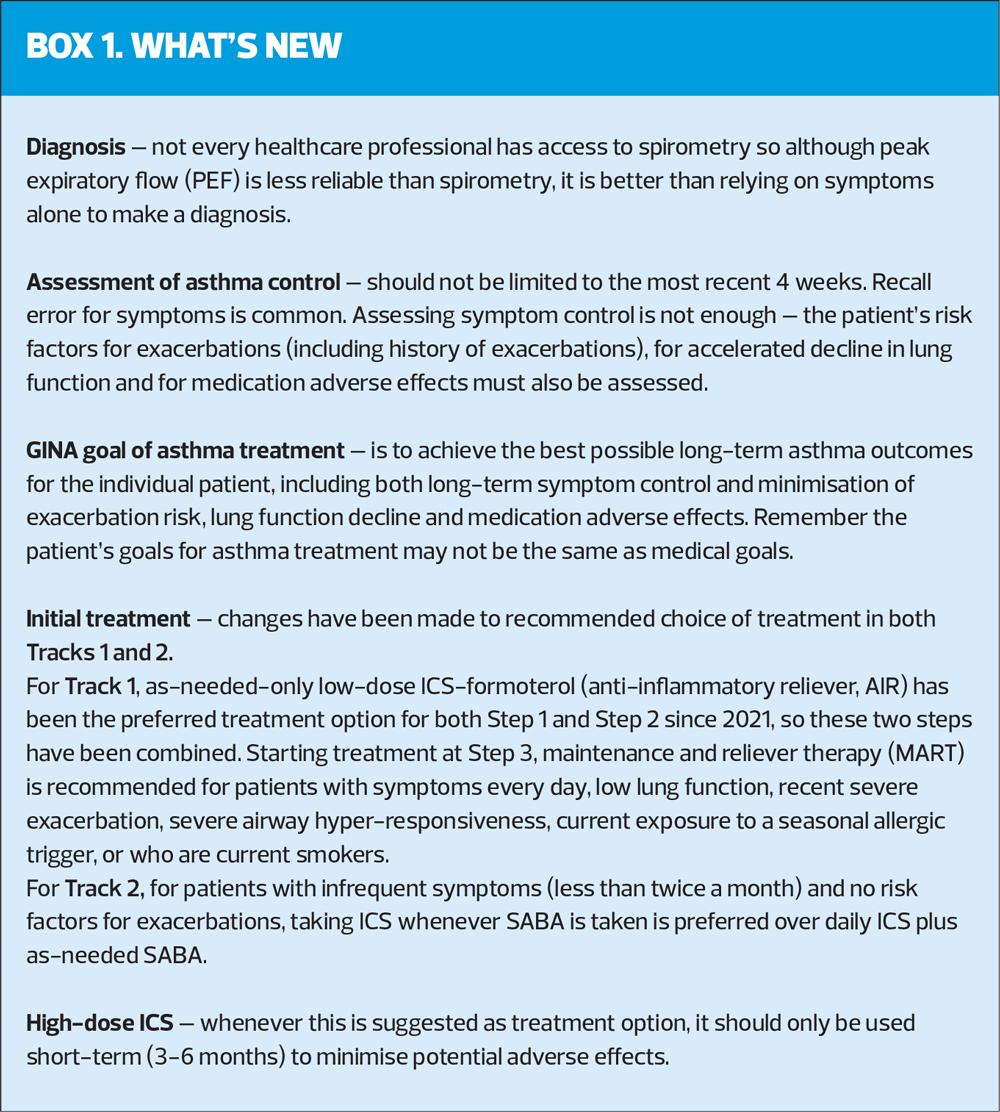
If healthcare professionals do not have access to spirometry, peak expiratory flow (PEF) should be used, rather than relying on symptoms alone. If using PEF, use the best of 3 measurements each time, and the same meter should be used for follow-up, as measurements may differ between meters by up to 20%.
Test before treating, wherever possible, as it often more difficult to confirm the diagnosis once asthma control has improved.
It is important to confirm the diagnosis to avoid unnecessary (or over-) treatment and to avoid missing other important diagnoses.
ASSESSMENT OF ASTHMA CONTROL
GINA defines the level of asthma control as the extent to which the features of asthma can be observed in the patient, or have been reduced or removed by treatment.
Control should be assessed in two parts – symptom control and the risk of adverse outcomes. Poor symptom control is onerous for patients and is associated with the risk of exacerbations – but that does not mean patients with good symptom control cannot have severe exacerbations.
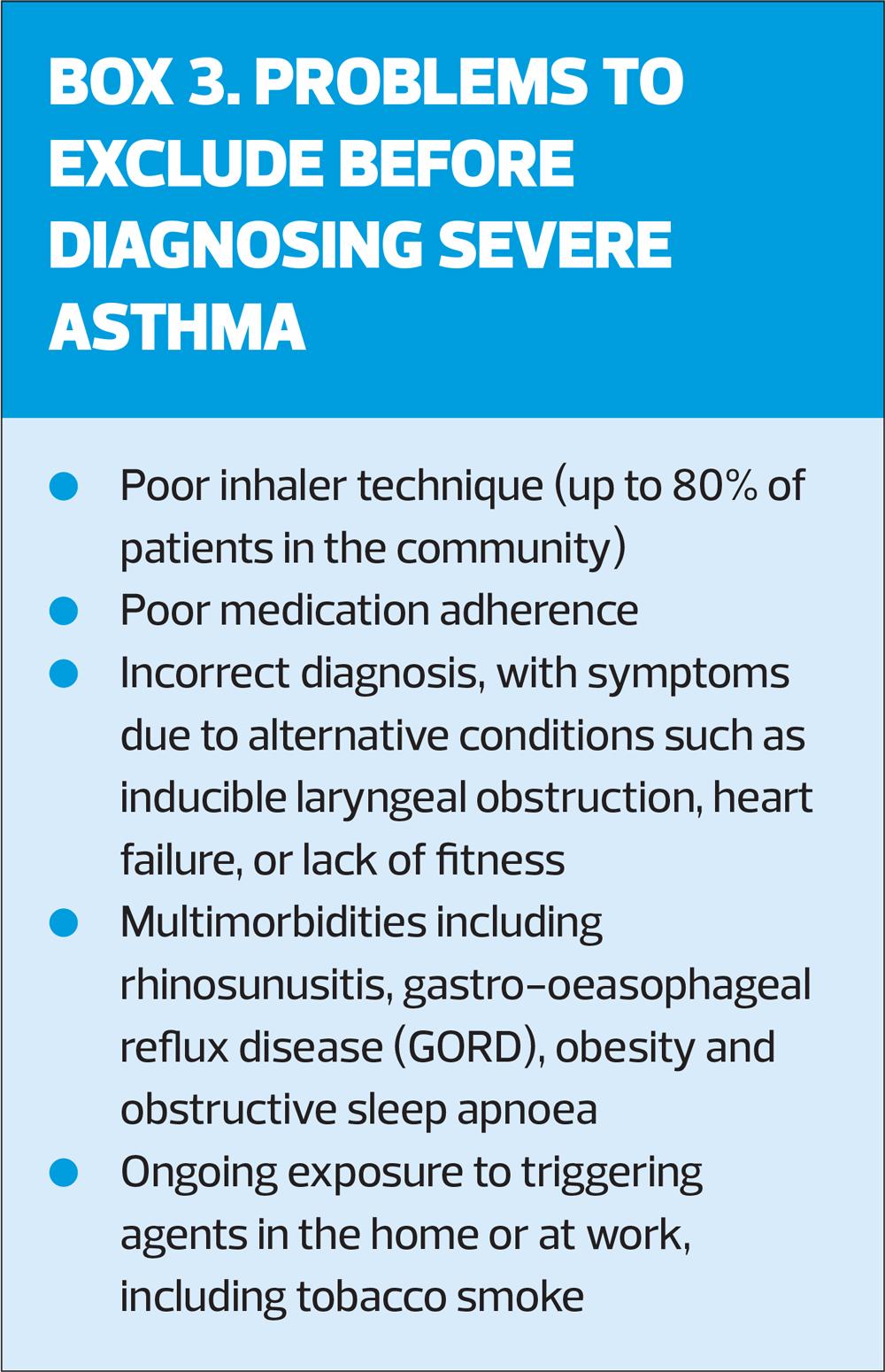
Asthma severity is assessed retrospectively, after at least 2–3 months of treatment, based on the intensity of treatment needed to control symptoms and prevent exacerbations. ‘Severe’ asthma identifies patients whose asthma is relatively resistant to high intensity treatment with high-dose ICS and a long-acting beta2 agonist (LABA), and who may benefit from biologic therapy. It is important to distinguish between severe asthma, and asthma that is uncontrolled due to poor inhaler technique/adherence (Box 3).
The retrospective definition of ‘mild’ asthma is less useful, as patients with only intermittent symptoms can still have an exacerbation, caused by viral infections or exposure to allergens, and the treatment that has been traditionally used as lowest intensity – SABA – can actually increase the risk of exacerbations. It is often assumed that patients with so-called mild asthma do not need ICS-containing treatment, but GINA has not recommended SABA-alone for any patient with asthma since 2019.
Symptom control should be assessed from the frequency of daytime and night-time symptoms, awakening at night, and limitation of activity, and for those patients using SABA, frequency of SABA use. The Asthma Control Test can be used to assess recent symptom control, but there are no validated tools for assessing symptom control over a longer period.
Separately, assess the patient’s risk factors for exacerbations, even if their symptom control is good. These include:
- History of ≥1 exacerbations in the previous year
- SABA-only treatment (without any ICS)
- Over-use of SABA (≥3 SABA inhalers/year)
- Socioeconomic problems
- Poor adherence
- Incorrect inhaler technique
- Low FEV1 (<60% predicted)
- Exposures such as smoking
- Raised blood eosinophils/high FeNO.
Also assess risk factors for persistent airflow limitation (preterm birth, chronic mucus hypersecretion), lack of ICS treatment in patients with a history of severe exacerbations, exposure to tobacco smoke, noxious chemicals, occupational or domestic exposures, and investigational findings such as low initial FEV1, sputum or blood eosinophilia.
Risk factors for medication side-effects should also be assessed, including frequent OCS, long-term high-dose ICS, P450 inhibitors, and poor inhaler technique.
GOALS OF TREATMENT
GINA has updated its goal for asthma treatment, which in essence is to ‘achieve the best possible long-term outcomes for the individual patient’. This includes:
- Good long-term symptom control (few/no asthma symptoms, no sleep disturbance and unimpaired physical activity)
- Minimised long-term risk of asthma-related mortality, with
No exacerbations
Improved or stable personal best lung function
No requirement for maintenance systemic corticosteroids
No medication side-effects.
The patient’s own goals should also be identified. Encourage patients (and caregivers) to participate in decisions about treatment. Consider the patient’s health literacy (the ability to obtain, process and understand basic health information).
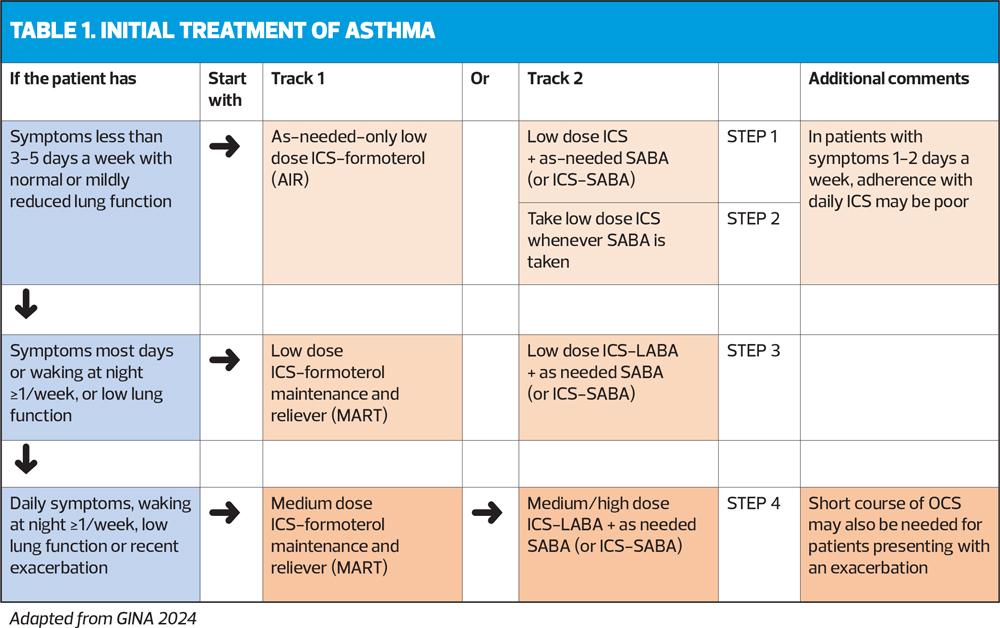
INITIAL TREATMENT
The 2024 report emphasises the importance of avoiding treatment of asthma (in adults, adolescents and children aged 6-11 years) with SABA alone, for safety reasons. Instead, patients should receive ICS-containing treatment to reduce their risk of serious exacerbations and to control symptoms.
ICS-containing treatment can be either regular daily treatment, or in those who have symptoms less than daily and normal or only slightly reduced lung function, with as-needed low-dose ICS-formoterol taken whenever needed for symptom relief.
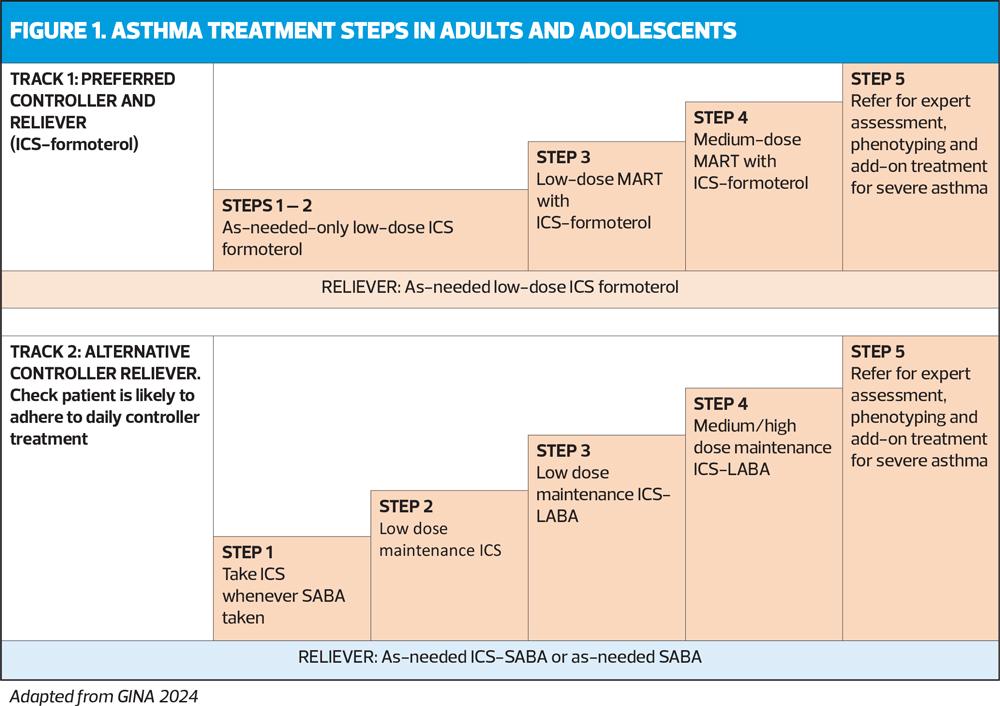
The treatment figure shows two ‘tracks’, based on the choice of reliever. Treatment can be stepped up or down within a track using the same reliever at each step, or switched between tracks, according to the individual patient’s needs.
Initial treatment steps are summed up in Table 1.
Track 1
In Track 1 (preferred), the reliever is low-dose ICS-formoterol. When a patient at any step has asthma symptoms, they use low-dose ICS-formoterol as needed for symptom relief. In Steps 3-5, they also take ICS-formoterol as regular daily treatment. This approach reduces the risk of severe exacerbations and provides similar symptom control, compared with using a SABA reliever. It also means that patients only need a single medication across Steps 1-4 (Figure 1).
Track 2
The reliever in Track 2 is an ICS-SABA or SABA, and can be used as an alternative if Track 1 is not possible, or if the patient is stable, with good adherence and no exacerbations in the past 12 months on current therapy. Before considering a SABA reliever, think about whether the patient is likely to be adherent with ICS-containing treatment: if not, they would be at higher risk of exacerbations. If reliever and maintenance medications are in different inhalers, ensure the patient can use both inhalers correctly.
Stepping up
Before stepping up treatment, confirm that symptoms are due to asthma, and address common problems such as inhaler technique, adherence, allergen exposure and multimorbidity.
The GINA-preferred Step 3 treatment is Track 1, low-dose ICS-formoterol maintenance and reliever therapy (MART), (Figure 1). This reduces the risk of severe exacerbations with similar or better symptom control, compared with using a combination ICS-LABA as controller, and as-needed SABA for symptom relief (Track 2). If patient needs a higher dose, it can be increased to medium by increasing the number of maintenance inhalations, e.g. from 1 inhalation twice daily plus 1 as needed, to 2 inhalations twice daily plus 1 as needed (See Medications and doses for GINA Track 1: AIR therapy, Box 4-8 in the 2024 report).
WHY ICS FROM THE START?
GINA recommends ICS-containing treatment from the moment a diagnosis of asthma is made, or as soon as possible thereafter, because:
- As-needed low-dose ICS-formoterol reduces the risk of severe exacerbations, A&E attendances and hospital admission by 65% compared with SABA-only treatment. The AIR regimen reduces severe exacerbation irrespective of baseline symptoms, lung function, exacerbation history or inflammatory profile.
- Starting treatment with SABA teaches the patient to rely on it as their main asthma treatment and increases the risk of poor adherence when ICS is subsequently prescribed
- Early treatment with ICS leads to greater improvement in lung function than if symptoms have been present for more than 2–4 years
- Patients who are not taking ICS who have an exacerbation have a greater long-term decline in lung function than those who are using ICS
- For patients with occupational asthma, early ICS treatment – together with removal of exposure to the trigger(s) – increases the possibility of resolution of symptoms and improvement of lung function/airway hyperresponsiveness.
CYCLE OF ASTHMA MANAGEMENT
Personalised asthma management means a continued cycle of assessment, adjustment of treatment, and review.
Assess
First, assess the patient’s symptom control and their risk factors for exacerbations, decline in lung function and medication side effects, paying attention to inhaler technique and adherence. Consider also comorbidities, the patient’s goals and preferences, and confirm the diagnosis if you have not already done so.
Adjust
Adjust the patient’s management, based on these individual assessments. Address modifiable risk factors – consider non-pharmacological strategies. Treatment of comorbidities should be optimised. Adjust asthma medication, including ICS, if required, and provide education and skills training. Update the patient’s personalised asthma action plan.
Review
Review the patient in line with the goals of treatment, reassess factors affecting symptoms, risk of adverse outcomes and patient satisfaction. Arrange further investigations if needed, and readjust treatment if needed.
Reference
Global Initiative for Asthma
2024 GINA main report, Global Strategy for Asthma Management and Prevention; 7 May 2024. https://ginasthma.org/2024-report/
Related guidelines
View all Guidelines