Eligibility criteria for contraception: UKMEC 2025
Mandy Galloway, Editor
Practice Nurse 2026;56(1):6-9
The latest update to the UK Medical Eligibility Criteria (UKMEC) for hormonal and emergency contraception, together with intrauterine devices, includes a raft of important changes that all clinicians need to be aware of to ensure safe and effective contraceptive care
UKMEC 2025 includes recommendations on a number of new conditions including multiple sclerosis, chronic kidney disease and sickle cell trait. There are also changes to recommendations for inclusion in UKMEC categories, with some conditions moving from low risk to higher risk categories. This article aims to provide a summary of the key changes but readers should refer to the full guideline in case of uncertainty regarding specific patients.
The College of Sexual & Reproductive Health (CoSRH, formerly the Faculty of Sexual & Reproductive Health, FSRH) reminds clinicians providing contraceptive care that UKMEC relates to safety, and not efficacy. Its recommendations apply to contraceptive use only – so, for example, if a method is used for another purpose such as controlling heavy menstrual bleeding, the criteria for use may be different. Conditions and characteristics that also increase a person’s risk of venous thromboembolism (VTE) have been reviewed throughout the UKMEC and categories have been upgraded
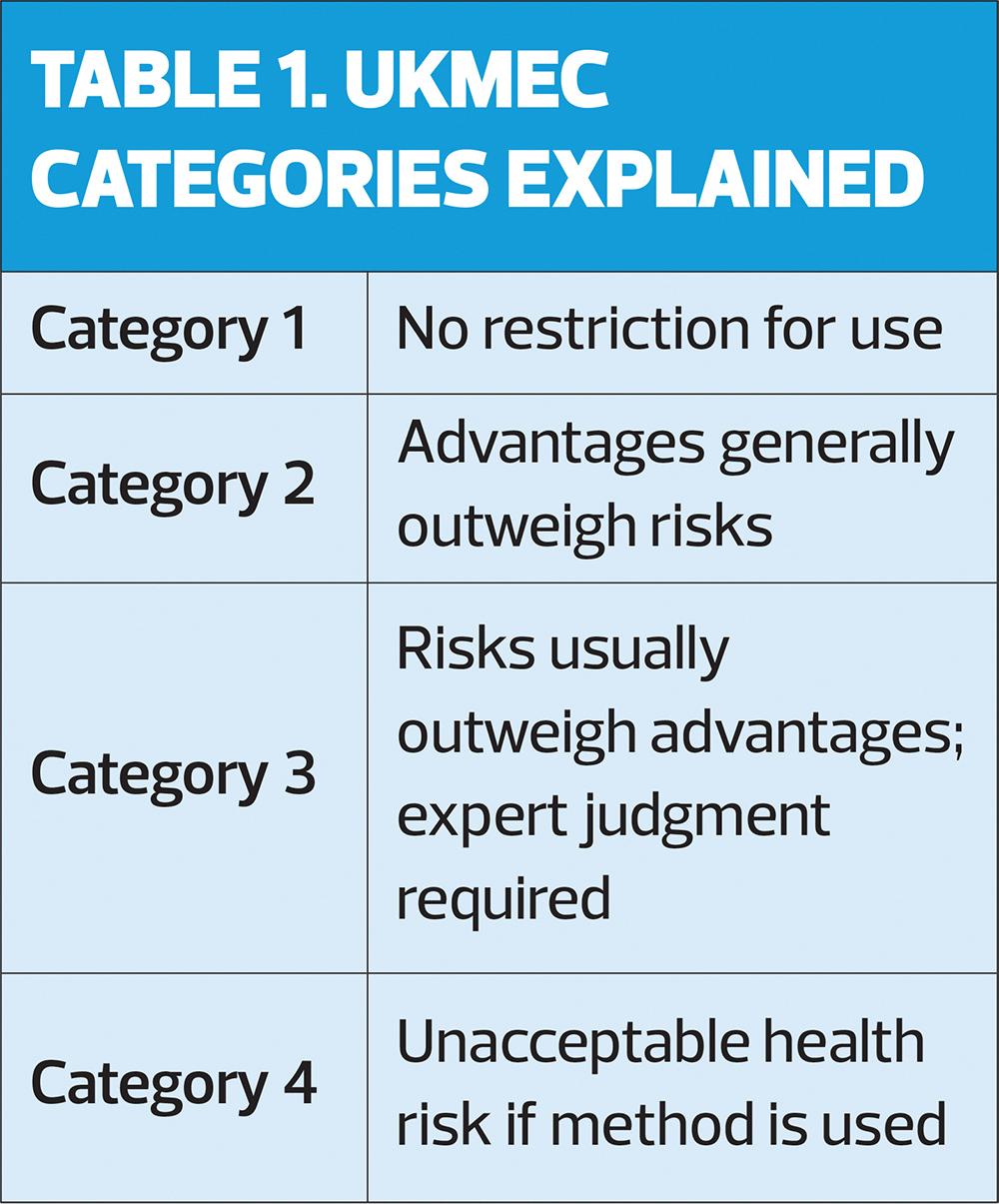
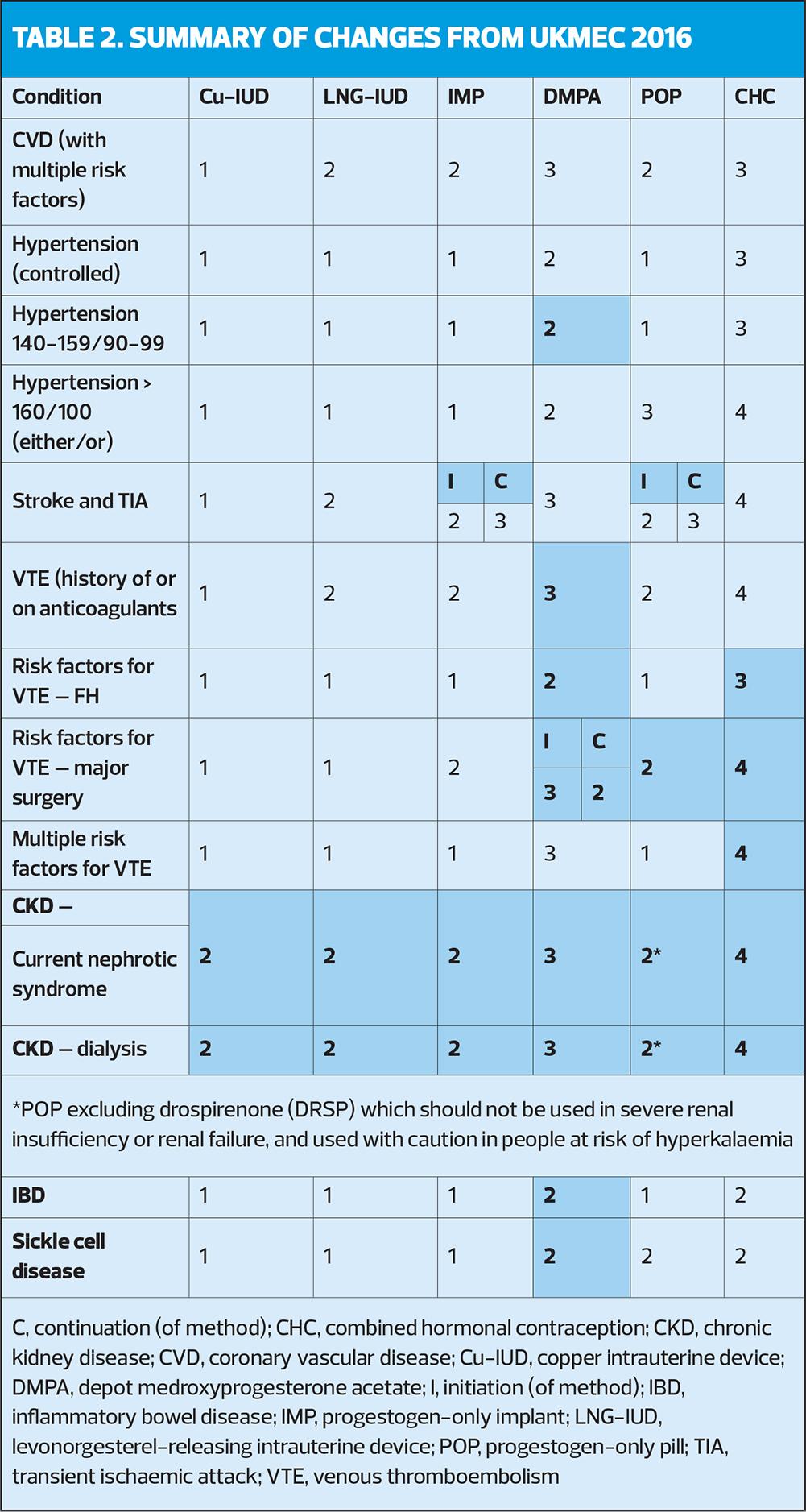
Importantly, clinicians are urged to remember that multiple UKMEC 2 categories may indicate cumulative risk and that they should use their clinical judgement. Multiple UKMEC 3 categories may pose an unacceptable risk. In this context, ‘multiple risk factors’ means ‘more than one’. Definitions of UKMEC categories are provided in Table 1. Key changes to risk categories in UKMEC 2025 are shown in Table 2.
NEW CONDITIONS
Multiple sclerosis (MS): Some evidence suggests that individuals with MS are at higher risk of VTE than those without MS, probably due to immobility, so it is important to differentiate between people with MS with prolonged immobility from those who are mobile. Patients with MS are also at increased risk of fractures.
Chronic kidney disease (CKD): All severities of CKD are associated with an increased risk of VTE, so combined hormonal contraceptives (CHC) are not a suitable option. Use of depot medroxyprogesterone acetate (DPMA) should be carefully considered because it has an adverse effect on bone health in patients with CKD,
Sickle cell trait: Sickle cell trait is associated with a modestly elevated risk of VTE; therefore, alternatives to combined hormonal contraception (CHC) should be selected.
Anxiety and mood disorders (formerly depression): Current research does not consistently show that hormonal contraceptives make anxiety or mood disorders better or worse in women who already have these conditions. When initiating hormonal contraception, clinicians should offer personalised counselling, encourage patients to keep track of their mood, and advise them to contact their healthcare provider if they notice any decline.
CONDITIONS THAT INCREASE HEALTH RISKS IN PREGNANCY
Long-acting reversible contraception (LARC) is recommended for women with conditions that increase health risks in pregnancy, including diabetes, hypertension, and some cancers.
INTRAUTERINE DEVICES (IUD)
Intrauterine devices (IUD) are highly effective, long-lasting and reversible methods of contraception. Their particularly low failure rate is due to the very minimal action required by the user. There are two types of IUD: the copper-bearing intrauterine device (Cu-IUD), and the levonorgestrel-releasing intrauterine device(LNG-IUD), formerly known as the LNG-intrauterine system to distinguish it from the ‘coil’.
There are several LNG-IUDs on the market, with varying doses of levonorgestrel, but the UKMEC guidance applies to all doses.
IUDs can be used in many conditions where oral contraceptive methods are contraindicated or not recommended, but initiation should be avoided in women with unexplained vaginal bleeding, with cervical cancer awaiting treatment, women being treated for breast cancer, or women with endometrial cancer. IUDs are not suitable for women with pelvic inflammatory disease or avsexually transmitted infection. In women with a distorted uterine cavity, expert advice should be sought.
IUD Q&A
Q. Can IUD be recommended for women with a history of VTE?
A. Yes, and they can be a useful treatment for heavy menstrual bleeding (HMB) in women on long-term anticoagulants.
Q. Can IUD be used in women with HMB?
A. LNG-IUD use causes changes in menstrual bleeding patterns, and users may become amenorrhoeic. The 52mg LNG-IUD can be used as a treatment for HMB.
Q. What about women with dysmenorrhoea?
A. Cu-IUD may intensify dysmenorrhea, including dysmenorrhoea associated with endometriosis. LNG-IUD may reduce dysmenorrhea.
Q. Can IUD be used in women with gynaecological cancer?
A. There is concern that insertion may increase the risk of infection and bleeding in women waiting for treatment for cervical cancer, so should not be initiated, but IUDs may be continued until treatment commences, to protect women at risk of pregnancy.
Q. What is advice should be given to women with blood disorders?
A. There is concern about increased blood loss with Cu-IUD in women with thalassaemia, sickle cell disease or iron-deficiency anaemia, but LNG-IUD is generally associated with reduced blood loss.
COMBINED HORMONAL CONTRACEPTION (CHC)
CHC is available as combined oral contraception (COC), transdermal patches, and vaginal rings.
UKMEC recommendations are based on low-dose COC containing ≤ 35μg ethyninylestradiol (EE). Data for newer COCs containing estradiol and estetrol are limited, so follow recommendations for EE-containing preparations.
After reviewing the limited evidence available for the combined contraceptive patch and vaginal ring, the UKMEC guideline development group concluded that these methods should be categorised as per COC.
Combined hormonal contraception: Q&A
Q. What should we do if BP increases in a woman with hypertension, current or history of ischaemic heart disease or stroke?
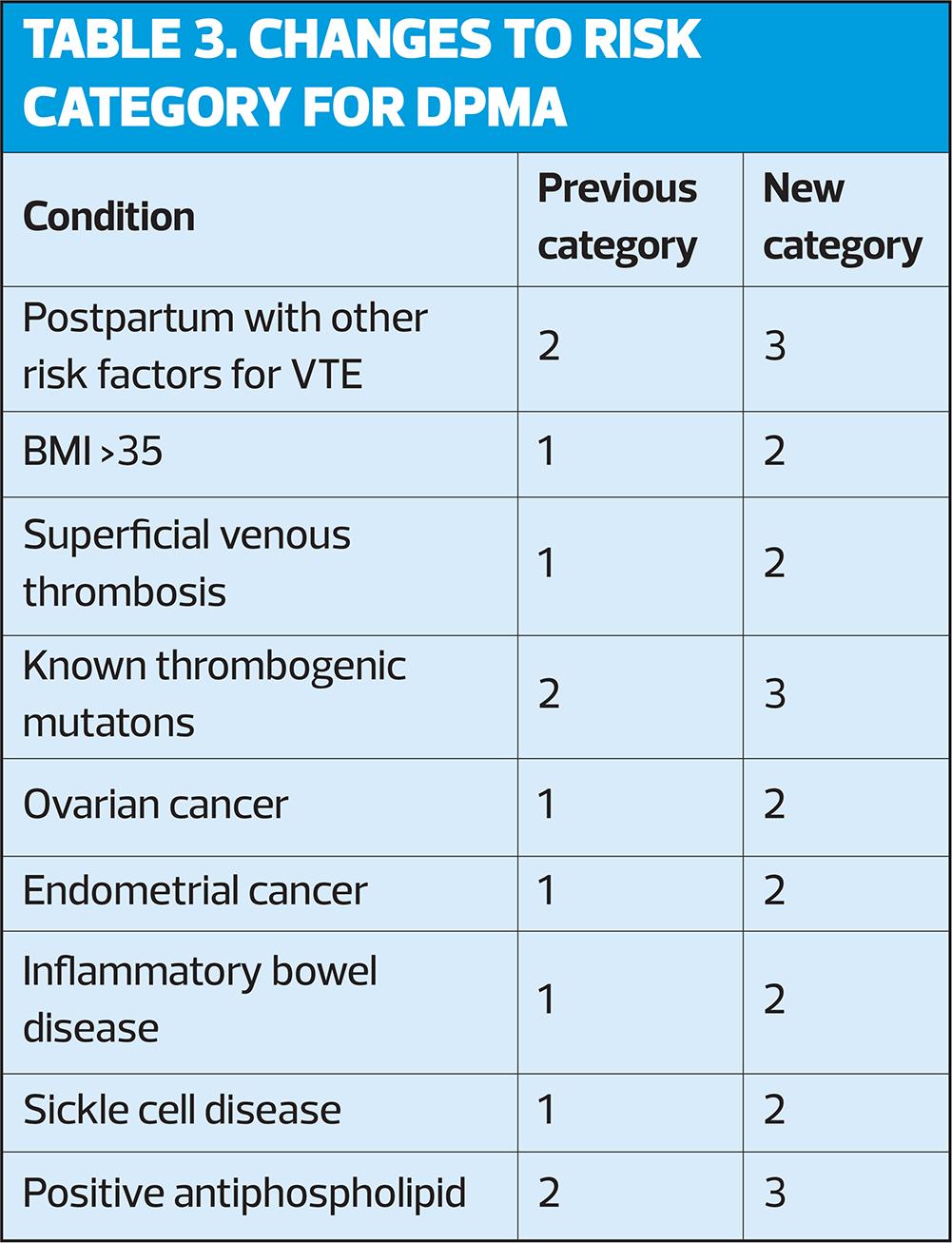
A. If BP is increased, it should be reassessed and monitored according to current guidelines. See Table 3 for UKMEC risk categories for women with these conditions.
Q. Should women with varicose veins be offered an alternative to CHC?
A. The presence of varicose veins is not a risk factor for VTE, but the risk of VTE is higher in women with superficial venous thrombosis.
Q. Can women with valvular and/or congenital heart disease, cardiomyopathy or cardiac arrhythmias be prescribed CHC?
A. Stasis, endothelial injury, and hyperviscosity (Virchow’s triad) increase the risk of clots. Impaired heart function, enlarged chambers, or arrhythmias contribute to stasis. Recent cardiac defect closure or a mechanical valve also increase thrombus risk.
Congenital heart disease: Surgical correction, co-existing complications and degree of cardiac disability will vary between individuals and should be considered when considering contraception use. The use of COC in women with these conditions represents an unacceptable health risk, and alternatives should be considered.
Q. Can CHC be used in women with unexplained vaginal bleeding?
A. No condition causing vaginal bleeding is aggravated in the short term by CHC use.
Q. What if the woman has endometriosis?
A. CHC does not worsen endometriosis, and may help to relieve symptoms
Q. Can CHC be used by women with fibroids?
A. There is no evidence that CHC affects the growth of uterine fibroids.
Q. Should women waiting for treatment for cervical, endometrial or ovarian cancer stop using CHC?
A. While there is a theoretical concern that CHC use may affect the prognosis of existing cervical cancer, women may continue to use CHC as the waiting time for treatment is likely to be short, and pregnancy would be contraindicated. COC reduces the risk of developing endometrial cancer, and can be continued while women await treatment, but should not be initiated at this point. In general the advantages of using the method outweigh the possible risks.
Q. Can CHC be used by women with liver disease?
A. COC are metabolised by the liver, and their use may adversely affect women whose liver function is compromised. In women with a history of cholestasis, COC-use may predict further episodes. COC may also worsen existing gallbladder disease.
Q. Should CHC be discontinued in women with iron deficiency anaemia?
A. No. CHC use may decrease menstrual blood loss.
PROGESTOGEN-ONLY CONTRACEPTION (POC)
POC includes the progestogen-only implant (IMP), the progestogen-only injectable depot medroxyprogesterone acetate (DMPA), and the progestogen-only pill (POP). Changes to risk categories for DMPA are shown in Table 3.
POC Q&A
Q. Is POC suitable for women with hypertension
A. For most women with hypertension – either controlled, or below 140-159/90-99 mmHg, the advantages of using POP outweigh the risks. For those with a BP of either 160 mmHg systolic, or 100 mmHg diastolic, the risks usually outweigh the benefits and expert advice should be sought. If BP is increased, it should be reassessed and monitored.
Q. Should POC be used for women with cardiovascular disease, ischaemic heart disease or stroke, or with diabetes?
A. There is some concern about hypoestrogenic effects and reduced HDL levels with the use of DMPA in women with these conditions but there is little concern about these effects with POP or IMP. NB, the effect of DMPA may persist for some time after discontinuation.
Q. Is it OK to use POC in women with unexplained vaginal bleeding?
A. POC may cause irregular bleeding patterns and mask symptoms of underlying pathology.
Q. Is POC suitable for women waiting for treatment for cervical, endometrial or ovarian cancer?
A. Yes – the period of waiting is likely to be short, and pregnancy would be contraindicated.
Q. Can women with liver disease use POC?
A. POC are metabolised by the liver, and their use may adversely affect women whose liver function is compromised. This concern is similar to, but less than, that with COC. Theoretically a history of COC-related cholestasis may predict subsequent cholestasis with POC use.
Q. Can POC be used by women with inflammatory bowel disease (IBD)?
A. The risk of VTE may be higher in women who are unwell undergoing surgery, or with prolonged immobilisation for IBD, but POC can be continued. Some studies have suggested that there is a risk of relapse among women with IBD using oral contraception, but most do not specify whether the method was a POP or COC, and the risk is not significantly greater than for non-users.
EMERGENCY CONTRACEPTION
Emergency contraception is a method that women of all reproductive ages can use to prevent unintended pregnancy following unprotected sexual intercourse (UPSI). The main methods are the Cu-IUD, which is the most effective, and oral EC – either ulipristal acetate (UPA) or oral progestogen-only EC containing levonorgestrel (LNG).
The Cu-IUD can be used from 0 to 120 hours of UPSI, or within 5 days of expected ovulation.
UPA can also be used within 120 hours of UPSI. LNG is only licensed for use up to 72 hours after UPSI or contraceptive failure. After 72 hours, efficacy may be reduced.
Emergency contraception: Q&A
Q. Can EC be used postpartum?
A. Yes. Women who are fully or almost fully breastfeeding, not having periods and <6 months postpartum can rely on lactational amenorrhea as contraception; if breastfeeding decreases or menstruation returns, EC may be indicated for UPSI.
Q. Can the Cu-IUD be used for EC in women with VTE?
A. Yes, but care should be taken when fitting a Cu-IUD in women taking anticoagulants because of the increased risk of bleeding.
Q. Can the Cu-IUD be used for EC in women with fibroids or a distorted uterine cavity?
A. The decision should be made on an individual basis, based on the degree of distortion, uterine cavity size and the availability of accurate imaging.
Q. Can EC be used in women with severe liver disease?
A. Because use of UPA or LNG is short-term, it is not expected to have as much clinical impact as longer term hormonal contraceptive use.
Q. Can Cu-IUD be used for emergency contraception in women at risk of sexually transmitted infection (STI)?
A. Yes, but women should be offered testing for STIs and given prophylactic antibiotics to prevent Chlamydia trachomatis at the time of insertion.
DRUG INTERACTIONS WITH HORMONAL CONTRACEPTION
Medications may affect the efficacy of hormonal contraceptives, requiring close monitoring.
- Healthcare providers should obtain a comprehensive medication history, including over-the-counter and herbal supplements.
- The effectiveness of DMPA and LNG-IUD is not affected by enzyme-inducing medications.
College of Sexual & Reproductive Healthcare (CoSRH). UK medical eligibility criteria for contraceptive use. UKMEC 2025; December 2025. https://www.cosrh.org/Public/Public/Standards-and-Guidance/uk-medical-eligibility-criteria-for-contraceptive-use-ukmec.aspx
Related guidelines
View all Guidelines