The 2017 GOLD guideline on COPD: implications for practice
Beverley Bostock-Cox
Beverley Bostock-Cox
RGN, MSc, QN
Nurse Practitioner Moreton in Marsh
Education Lead Education for Health, Warwick
UK national guidance on the diagnosis and management of COPD is seven years old. The GOLD 2017 update provides us with up-to-date, evidence-based guidance to inform practice and ensure that our patients get the best care
Last month’s journal included a summary of the comprehensively updated guidance on the diagnosis and management of chronic obstructive pulmonary disease (COPD) from the Global Initiative for Obstructive Lung Disease (GOLD).1,2 This advanced practice article discusses some of the key areas in more detail and considers how this affects general practice nurses (GPNs) working at an advanced level.
COPD is defined by the presence of symptoms, such as productive cough and breathlessness, and reduced capacity to carry out activities of daily living, with objective evidence of largely irreversible airflow obstruction. The main cause is exposure to noxious particles, which in the western world is predominantly cigarette smoke. COPD can have similar symptoms to other lung conditions such as interstitial lung disease (ILD) and non-lung conditions such as left ventricular systolic dysfunction (LVSD). It is therefore imperative to get the diagnosis right in order to ensure that people with COPD are offered the most appropriate management. Evidence-based practice, combining the findings from randomised controlled trials (RCTs) and real world evidence, should inform practice and ensure that people get the best care, both clinically and medico-legally.
The latest UK guidelines on managing COPD were published by the National Institute for Health and Care Excellence (NICE) in 2010.3 It is rumoured that an update of this guideline will be published in 2017. GOLD, however, produces annual guidelines, with the 2017 version published at the end of 2016. There are some significant changes regarding the definition, diagnosis and management of both stable and acute COPD of which GPNs, especially those working autonomously at an advanced level, should be aware.
DEFINITION AND DIAGNOSIS
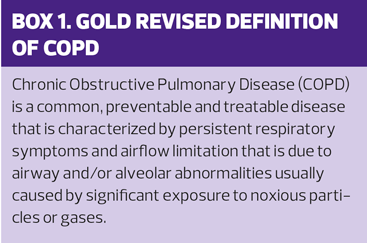
GOLD has updated its definition of COPD (Box 1) and more weight is given to the pathophysiological changes in airways and alveoli, the role of host factors (for example genetic influences, low birthweight and/or childhood respiratory illnesses) and the preventable causes, including smoking. The old definitions of chronic bronchitis (sputum for 3 months for at least 2 years) and emphysema as part of COPD are recognised as not being applicable to all patients, and the fact that many people at risk of COPD may have normal spirometry in the presence of respiratory symptoms is also highlighted.
Clinicians should suspect a diagnosis of COPD if a patient with a history of exposure to risk factors has:
- Progressive and persistent dyspnoea
- Chronic cough
- Persistent wheeze (expiratory and/or inspiratory)
- Chronic sputum production.
The probability of COPD is increased if there is a family history of the condition or a personal history of low birthweight or lung infections in childhood.
If the history is suggestive of COPD, post bronchodilator spirometry should be undertaken by a suitably competent individual. (See Activity 1) In a symptomatic individual this will usually show a reduced ratio of Forced Expiratory Volume in 1 second (FEV1) to Forced Vital Capacity (FVC) less than 0.7 (70%). The guidelines state that reproducible values are those within 5% and 150ml of each other. Many clinicians in the UK will be used to using a margin of 5% and 100ml, however.
GOLD also reviews the hotly debated subject of the use of the lower limit of normal (LLN) value rather than a fixed value for FEV1/FVC of 0.7 and comes down in favour of using the fixed valued rather than the LLN value for each patient.
NON-PHARMACOLOGICAL INTERVENTIONS
Before focusing on which inhalers to prescribe for people with COPD, GOLD recommends that consideration should be given to non-pharmacological interventions as these are often very effective and more cost effective than some inhaled therapies.
Pulmonary rehabilitation (PR) includes many of the most important elements of non-pharmacological management and, in particular, the focus is on diet, activity, relaxation and managing breathlessness. People should be encouraged to attend PR if it is available and the GPN can be an effective enthusiast of this approach. GOLD strongly supports the idea of helping people with COPD to develop their self-management skills and PR is set up to provide the knowledge, education and skills to facilitate this.
Other non-pharmacological treatments for COPD that are covered in the GOLD 2017 update include the appropriate use of non-invasive ventilation and lung volume reduction surgery.
Although flu vaccinations and smoking cessation both count as a pharmacological therapies, the role of smoking cessation as one of the most cost effective interventions for people with COPD should not be overlooked. GOLD endorses the use of nicotine replacement therapy and varenicline for smoking cessation but reserves judgement on the use of e-cigarettes.
THE ‘ABCD’ ALGORITHM
According to GOLD there are two main aims when treating COPD, one short term and one long term. The short-term aim is to reduce symptoms such as cough and breathlessness and the longer-term aim is to reduce risk e.g. the risk of exacerbations and the risk of dying.
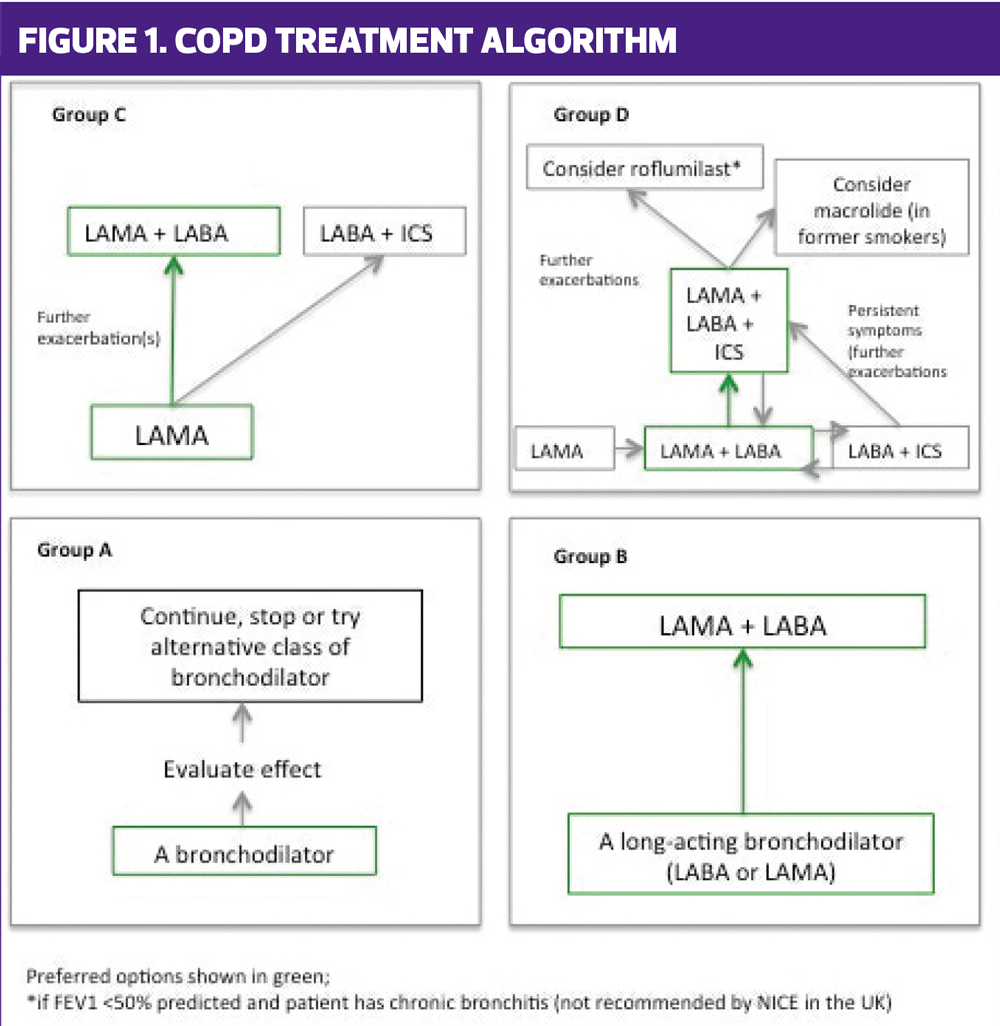
Acute exacerbations of COPD (AECOPD) are associated with further (and sustained) reductions in lung function and an increased risk of dying,4 so anything which reduces the risk of AECOPD can also be extrapolated to reducing the risk of death. According to the ECLIPSE study,5 the patients most at risk of having exacerbations are those who are already having them and studies suggest that these people are more likely to have the best risk: benefit ratio from being treated with inhaled corticosteroids (ICS) combined with a long acting beta2 agonist (LABA).6 In patients who are symptomatic with cough and breathlessness but who are not having 2 or more exacerbations per year, GOLD suggests that a bronchodilator may be the best therapeutic option – either a short acting beta2 agonist(SABA) or LABA, or a long acting muscarinic antagonist (LAMA), or both long acting versions given together. (Figure 1)
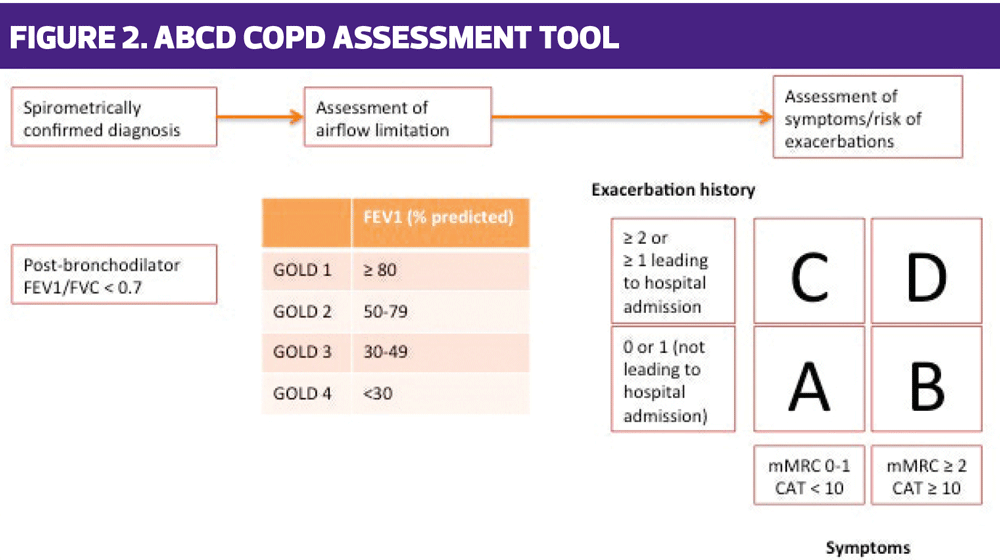
The GOLD guidelines include the ABCD algorithm (Figure 2), which allows a ‘phenotype’, based on the symptom burden and risk, i.e. exacerbation history, to be identified. In previous versions of this algorithm, lung function (specifically the FEV1) was included, but the current update has removed this element in recognition of the fact that lung function has very little correlation to the severity of COPD and is a less useful way of tailoring treatment than using symptoms and exacerbation risk. This approach is very different from that taken in the NICE COPD guideline where the focus is very much on the FEV1 as a key factor in pharmacological management.
The GOLD ABCD algorithm uses the modified Medical Research Council (mMRC) score (European version, scored from 0-4 rather than the UK’s 1-5) or COPD Assessment Test score (www.catestonline.org) as an indicator of symptom burden and then factors in the number of exacerbations suffered in the past 12 months to determine which category the patient belongs to: A, B, C or D. The guidelines then give examples of which treatment to use based on the relevant category.
- Category A patients: have a low symptom burden and a low risk of exacerbations – in fact they may not even be diagnosed as yet. GOLD does not advocate screening people for COPD but the guidance does encourage early diagnosis if people present with symptoms. Category A patients will normally just need a short acting bronchodilator but long acting versions may also be used.
- Category B patients: are symptomatic but not exacerbating and dual bronchodilators (a LABA with a LAMA in one inhaler) are offered first line to people in this category. They are also suggested as an add-in for people in other categories.
- Category C patients: are unusual and not often seen as they allegedly have a low symptom burden with regular exacerbations. A LAMA may well be most appropriate as these have been shown to reduce exacerbations and GOLD recommends the use of a dual bronchodilator over an ICS/LABA because of the risk: benefit ratio.
- Category D patients: are very symptomatic and have regular exacerbations so will also benefit from a dual bronchodilator and maybe even triple therapy with an ICS/LABA and a LAMA. Triple therapy inhalers containing all 3 drugs in one device are coming to the market imminently.
It is currently thought that too many people are on combination therapies (inhaled ICS and LABAs) who do not have a history of exacerbations and who may be being exposed to the possible risks of ICS (including pneumonia,7 and diabetes, especially where high dose ICS such as fluticasone proprionate 500mcg bd are used,8) without the benefits, which studies tell us are primarily in preventing further exacerbations in people with increased levels of eosinophils.7
INHALED THERAPY OPTIONS
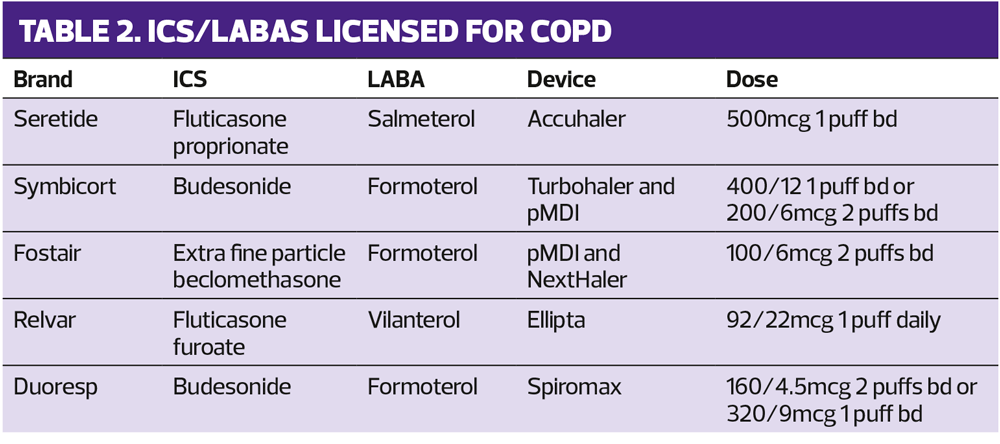
SABAs include salbutamol and terbutaline and the available short acting muscarinic antagonist is ipratropium. LABAs include salmeterol, formoterol and indacaterol. LAMAs include tiotropium, aclidinium, glycopyrronium and umeclidinium. ICS drugs licensed to use in combination with a LABA for COPD include fluticasone proprionate, budesonide, extra fine particle beclomethasone, and fluticasone furoate.
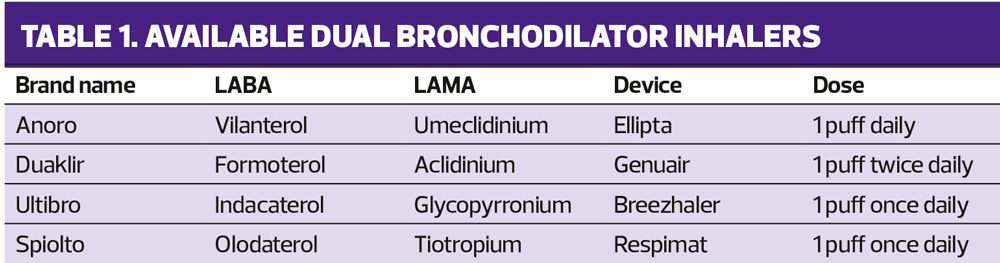
Dual bronchodilators have the potential to offer more than a single bronchodilator alone in COPD because the two classes of bronchodilators, LABAs and LAMAs, work on different pathways and have different modes of actions. LABAs facilitate bronchodilation via the sympathetic nervous system whereas LAMAs prevent bronchoconstriction via the parasympathetic nervous system. These two complementary actions allow for maximum bronchodilation, as well as reduced lung hyperinflation in patients with COPD resulting in improvements in breathlessness and cough.
The four available dual bronchodilators are shown in Table 1 and ICS/LABA combinations licensed for COPD are shown in Table 2.
In many cases, the decision as to which product to use will be based around the delivery system – in other words the patient’s ability to load and inhale from the device – and frequency of dosing. GOLD recommends that inhaler technique is taught and assessed throughout the course of treatment. Sometimes decisions may be informed by the recommendations on local formularies, but GOLD stresses the importance of tailoring care to the individual to provide a personalised approach, which reflects both the category and the person with COPD.
ACUTE EXACERBATIONS
AECOPD are defined by worsening of the usual COPD symptoms of breathlessness and sputum production, which require an upscaling of the patient’s normal treatment.
Eight out of ten AECOPD can be managed at home. First line would be to maximise bronchodilator use – SABA or SAMA (the latter only if the patient is not already taking a LAMA). Oral steroids can improve symptoms of dyspnoea and should be given at a dose of 40mg for 5 days. The need for antibiotics can be measured by identifying the presence of increased breathlessness, increased sputum production and increased purulence of sputum. Two of these may be enough, especially if the patient is unwell. Antibiotics should be given based on local guidance reflecting patterns of susceptibility and resistance and a 5-7 day course should be enough.
CO-MORBIDITIES
Many people with COPD will have, or be at risk of, other conditions such as cardiovascular disease, heart failure, diabetes, mental health problems (anxiety and depression) and osteoporosis from steroid use. GPNs should be aware of this and should aim to support people with COPD to reduce the risk of any co-morbidities. This should include regular assessment of mental health and the use of fracture risk assessment tools for patients on high dose ICS or regular oral steroids, as well as having an increased awareness of the fact that people with COPD are at higher risk of type 2 diabetes and cor pulmonale and may need assessment for these conditions.9
An often overlooked co-morbidity of COPD is sexual dysfunction, either due to breathlessness or due to problems with erections, which may have a significant effect on mood and quality of life.10 GPNs should be prepared to broach this subject with patients.
For those with co-morbidities, these should be managed holistically aiming for the best all-round health of the individual concerned. The message from the GOLD update is that co-morbidities should be treated the same in people with COPD as in those without. GOLD points out that COPD itself is an important co-morbidity, making the management of other conditions more challenging.
SUMMARY
GOLD guidance offers a pragmatic but evidence based approach to patient centred care for people with COPD. The latest update is a more comprehensive update of the guidelines and reflects current thinking about diagnosis and management of both stable and unstable COPD, along with assessing and treating people with co-morbidities.
GPNs should remember the importance of taking a structured approach to making a diagnosis of COPD and should be able to identify the most appropriate pharmacological and non-pharmacological interventions in order to improve outcomes for that patient, both in stable COPD and in acute exacerbations. GPNs working at an advanced level of practice need to be aware of the recommendations within these guidelines and should consider whether their current management is in line with best evidence.
REFERENCES
1. Galloway M. Guidelines in a nutshell: COPD: GOLD 2017. Practice Nurse 2016;46(12): 10-12. http://www.practicenurse.co.uk/index.php?p1=articles&p2=1408
2. Global Initiative for Chronic Obstructive Lung Disease (GOLD) (2017). http://www.goldcopd.org/
3. National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. CG101, 2010.
https://www.nice.org.uk/guidance/CG101
4. Schmidt SAJ, Johansen MB, Olsen M, et al. The impact of exacerbation frequency on mortality following acute exacerbations of COPD: a registry-based cohort study. BMJOpen 2014 http://bmjopen.bmj.com/content/4/12/e006720.full
5. Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010;11:122.
6. Spencer S, Evans DJ, Karner C, Cates CJ. Inhaled corticosteroids versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011; (10) CD007033
7. Ernst P, Saad N, Suissa S. Inhaled corticosteroids in COPD: the clinical evidence. European Respiratory Journal 2015 45: 525-537 Available at http://erj.ersjournals.com/content/45/2/525
8. Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. The American Journal of Medicine 2010;123:1001-1006 Available at http://www.amjmed.com/article/S0002-9343(10)00648-0/pdf
9. Rogliani P, Luca G, Lauro D (2015) COPD and diabetes COPD Research and Practice 2015; 1:3 https://copdrp.biomedcentral.com/articles/10.1186/s40749-015-0005-y
10. Collins EG, Halabi S, Langston M. et al. Sexual dysfunction in men with COPD: impact on quality of life and survival. Lung 2012 Oct;190(5):545-56
Related articles
View all Articles