The impact of Human Papilloma Virus on cervical screening
Val McMunn
Val McMunn
Visiting lecturer, City, University of London,
Clinical practitioner in contraception and sexual health, and independent trainer
The ability to detect High Risk Human Papilloma Virus (HR-HPV) has changed the way that cervical samples are tested and interpreted, and implementation of HPV testing into cytology screening has implications for practitioners, patients and laboratories in 2019 and beyond
The link between Human Papilloma Virus (HPV) and cervical cancer has been known for many years but it was not until the introduction of liquid based cytology that this virus could be isolated on a cervical sample. This has revolutionised the way the sample is interpreted and is the driving force for the changes in screening that are occurring.
Cervical screening became available in the 1960s, although the taking of samples at this time was very ad hoc. It was not until 1988 when the NHS Cervical Screening Programme (NHSCSP) was introduced that there was a structured approach and since then it is estimated that 4,500 lives have been saved each year in England alone.1
In the years 2014 to 2015 approximately 3.1 million women were screened in England, although nearly 4 million were invited for screening.2 The first request letter is sent to the woman at age 24.5 years, though there is often media excitement that the age of first sample taking should be lower. The age of first sample taking has been debated and thoroughly researched and 24.5–25 years is still recommended as the age of first sample.3 The rationale for this age is that young women are often exposed to the HPV and have associated cellular changes but these often regress spontaneously with time and without treatment.4 To detect these changes too early may lead to unnecessary treatment that may have a significant impact on the woman’s ability to maintain a pregnancy to term.5
However, the sad reality is that the highest numbers of non-attenders are in the age range of 25–35,6 and there are many interventions being developed to try to reduce this deficit. Non-attendance at the age of first request is putting young women at risk.
This short fall in first attendance may explain the fact that in 2014, 3,244 new cases of cervical cancer cases were found and over half (52%) of these were women under 45 years old. In the same year 890 women died of cervical cancer.7 The survival rate of cervical cancer has improved, and 63% of women diagnosed now survive for 10 years or more. These figures highlight how the treatment of cervical cancer has improved over time.
VACCINATION, HIGH RISK HPV AND CERVICAL SCREENING
Gardasil was introduced as the HPV vaccination of choice for girls aged between 11 and 14 in September 2012, and since 2014 girls are given two doses of the vaccine. The second injection is usually a year after the first but it can be any time between 6 to 24 months later.
Gardasil is a quadrivalent vaccine, and prevents abnormalities related to high risk HPV (HR-HPV) 16,18, and also HPV 6 HPV 11. HPV 6 and HPV 11(classified as low risk) are responsible for 90% of genital warts and are not related to cervical cancer.
If girls take up the vaccination at school, the programme will prevent at least 70% of cancers of the cervix, possibly even more in the future. As it takes between 10 and 20 years for a cancer to develop after HPV infection it is predicted that there will still be a rise in cervical cancer by 43% in the UK between 2014 and 2035, to 17 cases per 100,000 females by 2035 in women who have not been vaccinated.7 So any benefits in reducing cervical cancer won’t be seen for quite a long time. But the number of cases of pre cancerous changes in the cervix (cervical intraepithelial neoplasia [CIN]) will fall quite rapidly.
Three-yearly screening in younger women (five-yearly in older women) gives adequate time to detect and treat any abnormality before it develops into cancer.8
HR-HPV 16,18 AND CERVICAL CANCER
Infection with HR-HPV 16 and 18 significantly increases the risk of developing cervical cancer. Studies have shown that as many as 80% of the population who have had sex have at least one HPV virus by the age of 50 years old. HPV 16 is the most common HP virus, prevalence peaks early after onset of sexual activity.9 One in three women will develop a HPV infection within 2 years of starting to have regular intercourse, and about four out of five will develop the infection at some point in their lives.
As these statistics show, not everyone with the virus will develop cancer and the aetiology is poorly understood though the HPV 16 is implicated in nearly 100% of cervical cancer, 15% of oropharyngeal tumours and 60% of head and neck tumours.10
Although HPV is critical to the transformation of cervical epithelial cells into cancer, it is not sufficient on its own, and a variety of cofactors and molecular events influence whether or not cervical cancer will develop.11
Co-factors
Women whose immune system is depleted are at greater risk of developing cervical cancer if they have the high-risk virus. This includes women who have HIV, or have undergone an organ or stem cell transplant. Public Health England12 gives clear guidance for sample-taking in these situations, and women with HIV should have yearly screening. Highly active antiviral therapy (HAART) to reduce HIV load may also reduce the viral load of HPV, although research is limited and inconsistent.12
The number of children a woman has also affects the risk of developing cervical cancer after HR-HPV infection: the more children a woman has, the higher the risk. Having two children increases the risk two-fold.13 This risk is thought to be related to a reduction in tissue folate brought about by pregnancy so reducing the ability of the cell to respond adequately against mutation.13
Women who smoke are twice as likely to develop cervical cancer as those who do not, possibly because of the effect of chemicals in tobacco on the cells of the cervix and reduction in lymphocytes.14 Women smokers are more likely to die after diagnosis and treatment for cervical cancer compared with non smokers.
Women need to be encouraged to stop smoking in relation to cervical cancer.
Taking the oral contraceptive pill for more than 5 years also increases the risk of cervical cancer two-fold but the cause is not known.
FIRST CHANGES IN SCREENING
The knowledge of the link between HR-HPV and cervical cancer has revolutionised cervical screening. In 2001 a pilot study was undertaken which showed that HPV triage enabled a rapid and appropriate referral for women with low grade abnormalities.15
In 2007, six sentinel sites began to carry out HR-HPV testing on all samples that showed low grade abnormalities on routine screening. This study included about 10% of women screened in that year. The sentinel studies showed the high negative predictive value of HR-HPV testing, i.e. if HR-HPV is not present even in cells which show low grade changes the woman can be returned to routine screening with no higher risk of developing cervical cancer. Those who did have HR-HPV would be referred to colposcopy as these were the women who were found to be at risk of further negative cell development.16 These studies also showed cost effectiveness in terms of quality of screening and years of life saved.17 Based on these findings, HPV triage and test of cure was rolled out in England in 2013.
In 2013 the roll out of HR-HPV triage was extended to women with borderline or low grade cytology results. This meant that any women with low grade abnormalities would automatically be HPV tested. If no HR-HPV was detected, the woman would go back to normal recall; if HR-HPV was present then the women would be sent to colposcopy for further investigation/ treatment,12 as these were the women who were at risk of developing cervical cancer.
HR-HPV testing was also introduced as a test of cure after colposcopy to determine the need for further treatment and appropriate screening.12 Women who were reported as high grade dyskaryosis or worse are automatically referred for colposcopy without a HR-HPV test as treatment is obviously required. This method of triage was found to be acceptable for women who preferred immediate referral as opposed to further cervical surveillance.18
After having been given treatment for any grade of CIN women are screened again in 6 months. This sample can be taken in the community. This test is screened for abnormal cells and HR-HPV. If the HR-HPV is negative the woman will be screened in 3 years no matter what her age. This applies whether the result for the cells is negative, borderline changes in squamous endocervical cells or low-grade dyskaryosis. A positive HR-HPV result means automatic referral to colposcopy.
FURTHER CHANGES TO SCREENING
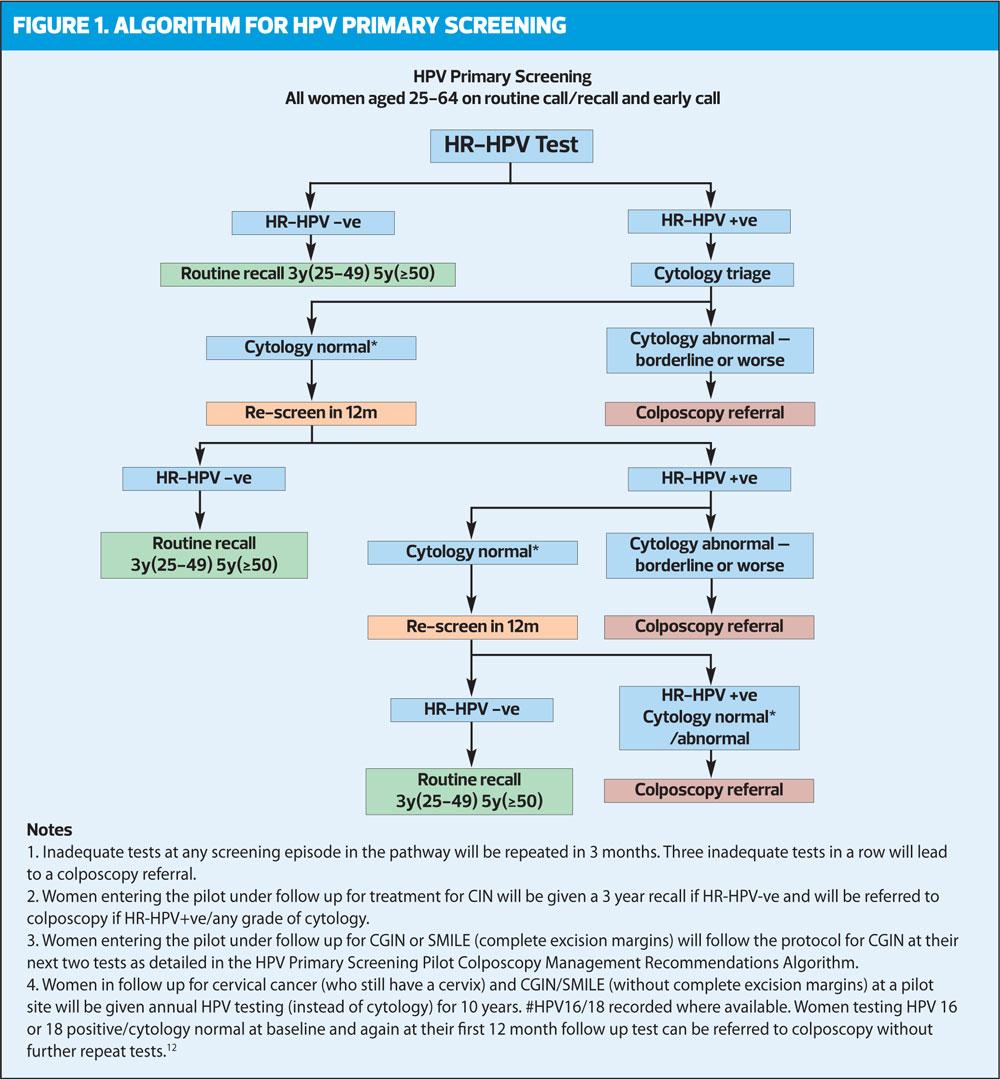
The success and effectiveness of the above studies formed the basis of the development and roll out of the use of HPV testing as primary screening and this will be fully implemented in 2019.
This means that the cervical sample is taken from the woman as usual but once in the lab the HPV primary screening differs from the usual process for examining cervical samples. Instead of the sample being examined under a microscope by a cytoscreener for any signs of abnormality, it is first tested for HR-HPV. If the sample is found to be HR-HPV-negative, the woman is returned to routine recall and invited for screening again in three or five years’ time depending on her age and the cells are not examined. If the sample is HR-HPV positive, a slide is prepared from the same sample and is examined by the cytoscreener for any abnormal cells.
As women can test HPV positive with no changes to the cells this cytology check ensures that these women are not referred unnecessarily for further investigation. However, they are kept under observation by having samples taken more frequently – annually for 3 years. If still HR-HPV positive, they would be sent for colposcopy.
HR-HPV testing identifies more abnormalities than cytology testing, and can even identify a sample that is normal except for a single dyskaryotic cell. If this cell had not been collected/seen the sample would have been processed as ‘normal’. HPV primary screening may therefore be a better way of identifying women at risk of developing cervical cancer.
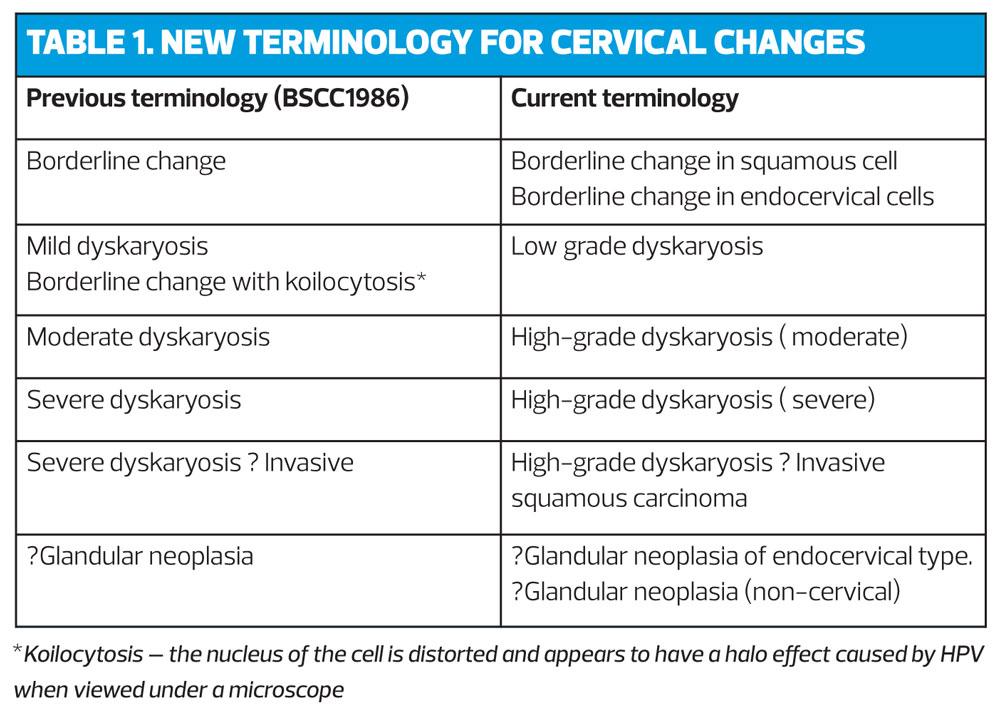
This screening programme has been devised because what has been proven is that persistent infection with a high-risk human Papilloma virus (HPV) genotype is required for the development of high-grade cervical neoplasia (cervical intraepithelial neoplasia [CIN] grade 3, adenocarcinoma in-situ, and invasive cervical cancer (CIN3 +).19 The new terminology for describing abnormalities can be seen in Table 1.
It must be remembered that cancer of the cervix takes many years to develop (10-20 years) having gone through the changes of CIN, and the detection of HR- HPV shows a higher risk of cancer and is not an absolute for development. Primary screening with HR-HPV testing generally detects more than 90% of all cases of CIN2, CIN3, and invasive cancer. HR-HPV testing is approximately 25% more sensitive than liquid-based cytology in detecting borderline changes though it is about 6% less specific.20 Guglielmo et al21 also found HPV-based screening is more effective than cytology in preventing invasive cervical cancer, by detecting persistent high-grade lesions earlier and providing a longer low-risk period. However, in younger women, HPV screening leads to over-diagnosis of regressive CIN2.21 This may not be such a risk when the primary screening is of women who have been vaccinated against HPV. Further development in HPV testing with separate HPV16 and HPV18 detection could provide an alternative, more sensitive, and efficient strategy for cervical cancer screening than do methods based solely on cytology.22
POSSIBLE CHANGES TO COME?
A negative HR-HPV result at baseline predicts one-half the risk of CIN3+ over 3 years compared with a negative cytology result. These findings may mean that the screening round may be longer between cervical screenings for women who are HR-HPV negative.20,22 In future, these women may possibly only need screening after a new sexual partner.
Women who have been vaccinated are less at risk and their screening round may need to be reviewed as the findings develop in relation to cytology outcomes.
A study in 2010 showed that women who were persistent non attendees for cervical screening found self swabbing for HPV acceptable. If the swab highlighted HR-HPV and showed the higher risk the women had for developing CIN they were then more likely to attend for cervical screening.23 This primary screening detects more than 90% of all CIN2, CIN3 and invasive cancer.20
Self swabbing for HR-HPV has been shown to be acceptable to women, and given the persistently low rate of attendance for cervical sample taking, postal kits or self swabbing in clinics for HR-HPV may be a way to highlight possible risk and encourage the woman to attend for cervical screening.
The success – in the long term – of the HPV vaccination programme may mean that the need for cervical screening is eventually relegated to the history books, and healthcare professionals in years to come will look back and wonder what all the fuss was all about.
CONCLUSION
The ability to detect HR-HPV from a cervical sample and the knowledge of its impact on the development of cervical cancer has caused a revolution in the screening of cervical samples. Primary screening for HPV has been found to be safe for women, cost effective and will continue to impact on cytology screening in this country. HPV holds the key to further developments and treatment to further reduce cervical cancer mortality.
REFERENCES
1.Peto J, Gilham C, Fletcher O, Matthew F. The cervical epidemic that screening has prevented in the UK. Lancet, 2004, 364:249-56
2.Cervical screening programme, England – Statistics for 2014-15. The health and social care Information Centre, November 2015
3.Sasieni P, Caston A, Cuzick J. Effectiveness of cervical screening with age:population based case- contol study of prospectively recorded data. BMJ 2009,339:b2968
4.Sasieni P, Caston A, Cuzick J. Impact of cervical screening on young women: a critical review of the literature ( NHS CSP Publication No 31) Sheffield: NHS Cancer Screening Programmes, 2010
5.Jakobsson M, Gissler M, Paavonen J. Loop electrosurgical excision procedure and the risk for preterm birth. Obstetrics and Gynaecology, 2009;114(3):504-10
6.Jo’s Trust. Body shame responsible for young women not attending smear tests. Press release, 22 January 2018. https://www.jostrust.org.uk/node/1073042
7.Cancer Research UK. Cancer statistics by cancer type: cervical cancer http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/cervical-cancer
8.Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003;89(1):88-93
9.St Guily JL, Clavel C, Okais C, et al. Human Papilloma Virus genotype distribution in tonsil cancers. Head Neck Oncol 2011;3(1):6. doi: 10.1186/1758-3284-3-6.
10.Gillison M: HPV and its effect on head and neck cancer prognosis. Clin Adv Hematol Oncol 2010;8(10):680-682
11.Burd EM. Human papillomavirus and cervical cancer/ Clin Microbiol Rev 2003;16:1-17
12.Public Health England. NHS Cervical Screening Programme. Colposcopy and programme management. NHSCSP publication number 20, March 2016. https://www.gov.uk/government/ uploads/system/uploads/attachment_data/file/515817/NHSCSP_colposcopy_management.pdf
13.Piyathilake CJ , Macaluso M , Johanning GL, et al. Methylenetetrahydrofolate reductase (MTHFR) polymorphism increases the risk of cervical intraepithelial neoplasia. Anticancer Research 2000:20(3A):1751-1757
14.Fonseca-Moutinho JA. Smoking and cervical cancer. ISRN Obstet Gynecol 2011;2011:847684 doi:10.5402/2011.847684
15.Moss S, Gray A, Legood R. et al. Effect of testing for human papillomavirus as a triage during screening for cervical cancer: observational before and after study. BMJ 2006:332:83–85
16.Arbyn M,Martin-Hirsch P, Buntinx F, et al Triage of women with equivocal or low – grade cervical cytology results: a meta- analysis of the PPV test positivity rate. J Cell Mol MED. 2009 April ;13 ( 4) :648-59
17.Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid- based cytology in primary cervical screening (ARTISTIC): a randomised controlled trail. Lancet Oncol 2009;10(7):672-682
18.Frederiksen M, Lynge E, Rebolj M. What women want. Women’s preferences for the management of low- grade abnormal cervical screening tests: a systematic view. BJOG 2012;119:7-19
19.Wright T, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: End of study results from the ATHENA study using HPV as the first-line screening test. Gynecologic Oncology 2015;136:189–197
20.Kitchener HC, Almonte M, Thomson C, et al. ARTISTIC ; a randomised trial of HPV testing in primary cervical screening. Health Technol Assess 2009;13 (51):1-150 iii-iv doi:10.3310/hta13510.
21.De Guglielmo Z, Rodriguez A. Methods used in the identification of HPV. An Sist Sanit Nar 2010;33:71-7 (in Spanish).
22.Dijkstra MG, van Zummeren M, Oozendaal L, et al. Safety of extending intervals beyond five years in cervical screening programmes with testing for high risk human papillomavirus: 14 year follow up of population based randomised cohort in the Netherlands. BMJ 2016;355:i4924.
23.Murat Gok, Heideman D, Kemenade R, Berkhof J. HPV testing on self-collected cervicovaginal lavage specimens as a screening method for women who do not attend cervical screening: cohort study. BMJ 2010:340:c1040
Related articles
View all Articles