Best practice: the updated UKMEC contraception guidance
Dr Caroline A Taylor
Dr Caroline A Taylor
BM, FFSRH
Associate Specialist and Clinical Lead for Sexual and Reproductive Health
Sexual Health Service, Solent NHS Trust
Fellow of Faculty of Sexual and Reproductive Health (FSRH),
Member of British Menopause Society (BMS)
Knowing which contraceptive method can be safely used by which woman is essential knowledge for all clinicians involved in women’s healthcare. Updated guidance from the Faculty of Sexual and Reproductive Healthcare reflects current evidence and following it will help you adhere to best practice
Many women using contraceptives are fit and healthy and the use of contraception therefore carries very low risk. However, some women have medical conditions or certain characteristics which increase the risks associated with some contraceptives. Knowing which methods are suitable for which women is essential knowledge for all clinicians prescribing contraception.1
In 1996, the World Health Organization (WHO) published a set of Medical Eligibility Criteria (MEC) designed to inform clinicians across the world about which contraceptives may be contraindicated in women with certain medical conditions. These were evidence-based and have been updated several times over the years. As these were designed mainly for use in developing countries, where the risks of pregnancy can be high, it was envisaged that other countries with lower risks could adapt this guidance. In 2006, the Faculty of Sexual and Reproductive Health (FSRH) published the first edition of the UK Medical Eligibility Criteria (UKMEC), and the second edition followed in 2009.
The third edition of the UKMEC was published in May 2016,1 following the latest WHO MEC guidelines. It is available to everyone on the FSRH website (www.fsrh.org). UKMEC 2016 includes some new conditions and removes others, removes guidance for certain contraceptives and clarifies certain risks in order to make it easier to use and more relevant to women living in the UK today.
HOW TO USE THE UKMEC
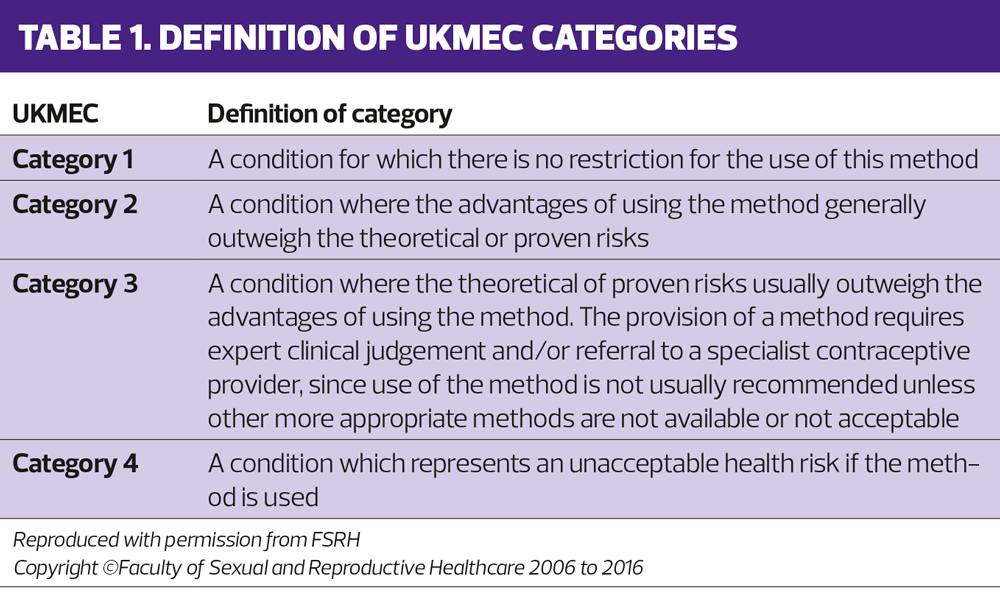
For each different contraceptive type (intrauterine contraceptives – IUD and IUS, progestogen only implants, progestogen only injections, combined hormonal contraceptives – pills, patches and rings, and progestogen only pills) each relevant medical condition is given a UKMEC category from category 1 to category 4 – shown in the table 1.
In practice, this means:
- Category 1: no problem – the contraceptive method can definitely be used
- Category 2: the method can be used but consideration needs to be given to the risks
- Category 3: generally the method should not be used. An expert opinion should be obtained before prescribing the contraceptive method
- Category 4: the contraceptive method should not be used.
These categories relate to the safety of the method when used in women with a particular condition or characteristic. They do not reflect the efficacy of that method with that condition and/or treatment.
It should be remembered that UKMEC refers to the use of the contraceptive method for contraception only. If the method is being used for another reason, e.g. management of menorrhagia, then the risk to benefit ratio may be different.
The other thing to note is that the categories are not additive. If a woman has 2 conditions or characteristics, which are UKMEC 2, this does not mean that the method is a UKMEC 4. However, if a woman has multiple UKMEC 2 conditions for one contraceptive method, the clinician should consider whether an alternative method would be more suitable.
Initiating and continuing methods
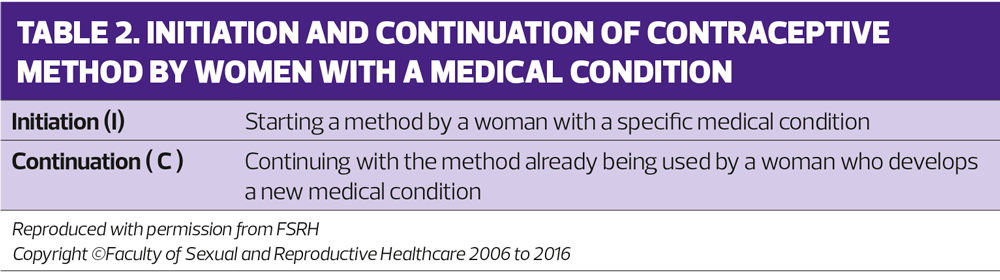
Sometimes the category an individual method is given will depend on whether the woman had the condition before that method was started (initiation) or whether the woman developed the condition whilst on the contraceptive method (continuation) – see table 2.1 The concern with continuation of the method is whether, theoretically, the method could have contributed to the development of the condition in question.
Clarification
For each medical condition or characteristic, clarification is given explaining the evidence.
HOW UKMEC 2016 DIFFERS FROM PREVIOUS VERSIONS
Contraceptive methods
The only contraceptive methods considered in the new 2016 UKMEC are:
- Intrauterine contraceptive methods – both the copper IUD (Cu-IUD) and levonorgestrel IUS (LNG-IUS)
- The progestogen only implant
- The progestogen only injectable
- The progestogen only pill (POP)
- Combined hormonal contraception (CHC).
Emergency contraception (EC) is also considered and has been updated to include Ulipristal Acetate (UPA). The order in which the EC methods are presented reflects their decreasing efficacy i.e. from Copper IUD to UPA to levonorgestrel (LNG).
Although the UKMEC 2016 recommendations for the progestogen only injectable are for depot medroxyprogesterone acetate (DMPA), either intramuscular or subcutaneously, it is stated that the categories are the same for intramuscular norethisterone enantate which, although unlicensed for long term use in this country, is used occasionally.
Barrier methods (male or female condoms, and diaphragms and caps), fertility awareness methods (including the lactational amenorrhoea method) and male and female sterilisation have not been included in this edition as they are dealt with in detail in their individual FSRH clinical guidance documents. These documents are available in the clinical guidance section of the FSRH website.
The order in which the method categories are presented has changed in order to reflect their length of action: from long to medium to short acting.
Medical conditions
In view of the fact that both malaria and schistosomiasis are very uncommon in the UK and that there is no evidence that hormonal contraception is contraindicated in these infections, they have been omitted from the new guidance.
It is now thought that in Raynaud’s disease the underlying disease process affects risk of contraception rather than the Raynaud’s itself, therefore this too has been omitted from the new guidance.
As more women in the UK are living with more complex medical conditions, the UKMEC has included new conditions in order to give clinicians guidance as to the suitability of the different contraceptive methods in these conditions. The following have therefore now been included:
- History of bariatric surgery
- Organ transplant
- Cardiomyopathy
- Cardiac arrhythmias
- Rheumatoid arthritis
- Positive antiphospholipid (aPL) antibodies.
Newer evidence and experience in clinical practice has led to certain sub-conditions being revised. These include:
- The post-partum period
- Gestational trophoblastic disease
- Cervical cancer
- Infection with human immunodeficiency virus (HIV)
- Systemic lupus erythematosus (SLE).
In UKMEC 2009, certain conditions had split categories e.g. UKMEC 2/3 or 3/4. This ambiguity was not helpful for many clinicians and has therefore been removed in this edition. Where necessary, clarifications have been highlighted to aid clinical judgement as to whether a method is safe and/or appropriate.
Drug interactions
Some medications interact with hormonal contraceptives. Some can decrease the efficacy of hormonal contraceptives and, conversely, some hormonal contraceptives can affect other medications causing adverse effects. Unfortunately, any documented evidence on drug interactions can quickly become out of date as new drugs are developed and new evidence is found.
Potential drug interactions are therefore no longer documented. Instead, where medications are known to interact with contraceptives (e.g. HIV, infection and epilepsy) there are reminders to check online drug interaction checkers (websites are documented) and/or the FSRH guideline on Drug interactions with hormonal contraception (available in the clinical guidance section of the FSRH website).
Diagnosis of migraine
In section C of the UKMEC 2016, there is a very helpful resource regarding migraine with aura. As those who currently work in the area of contraception can attest, the diagnosis of migraine with aura can be very difficult, especially in women with vague symptoms. These additional resources, including a link to a video showing how an aura presents and detailed descriptions of migraines with and without aura, are a welcome addition to the guidance.
SUMMARY OF THE IMPORTANT CHANGES
At the beginning of the UKMEC 2016 guidance there is a helpful summary of the changes that have occurred since the last edition. Below is a quick summary highlighting the main changes. General practice nurses should refer to the UKMEC 2016 for full details.
Post-partum period
Breast feeding women can now use CHC from 6 weeks to 6 months after delivery (UKMEC 2) as there has been no evidence that its use affects the outcome of the breast-fed child.
Non-breastfeeding women may be able to use CHC before 6 weeks post-delivery depending on whether they have any additional risk factors for venous thromboembolism (VTE). Risk factors include:
- Immobility
- Transfusion at delivery
- Body mass index (BMI) greater than or equal to 30
- Post-partum haemorrhage
- Immediately after caesarean section delivery
- Pre-eclampsia
- Smoking.
If a woman has one or more risk factors for VTE and is less than 3 weeks post-delivery, CHC is contraindicated (UKMEC 4), but this reduces to UKMEC 3 between 3 and 6 weeks post-delivery. In a woman who does not have any risk factors and who is less than 3 weeks post-delivery, CHC is UKMEC 3, reducing to UKMEC 2 between 3 and 6 weeks.
Cardiovascular disease
In women who have multiple risk factors for cardiovascular disease CHC is a UKMEC 3. If she just has a known dyslipidaemia, CHC is UKMEC 2. However, if they have a severe genetic lipid disorder, the use of CHC should be carefully evaluated.
Cardiomyopathy is a new addition to UKMEC. The category given depends on whether the woman has normal cardiac function – this can be assumed if the woman is not on any cardiac medication. In women who have cardiomyopathy but normal cardiac function, all contraceptive methods are UKMEC 1 except for CHC which is UKMEC 2. However, if a woman has cardiomyopathy and impaired cardiac function then all methods are UKMEC 2 except CHC, which is UKMEC 4.
Cardiac arrhythmias are another new addition. In atrial fibrillation CHC is contraindicated (UKMEC 4). Other contraceptive methods are UKMEC 2 except the Cu-IUD which is UKMEC 1. Known long QT syndrome can be a problem in women requesting intrauterine contraception. Fitting an intrauterine device stimulates the cervix and this may cause a bradycardia which, in the presence of long QT syndrome, may precipitate a cardiac event. Both the Cu-IUD and LNG-IUS are therefore a UKMEC 3 for initiation, falling to a UKMEC 1 for continuation of the method. CHC and DMPA are UKMEC 2, with the progestogen only implant and POP being UKMEC 1.
Bariatric surgery
This is a new section. Women who have had previous bariatric surgery can use any type of contraceptive freely (UKMEC 1) except for CHC. The use of CHC in the presence of bariatric surgery depends on their current BMI. If their BMI is less than 30, CHC is UKMEC 1, but this rises to 2 with a BMI of between 30 and 34, and to 3 if their BMI is 35 or more.
There are generally 2 types of bariatric surgery – procedures to reduce storage capacity of the stomach (gastric band, gastrectomy or gastroplasty) and procedures to decrease the absorption of nutrients by shortening the functional length of the small intestine (Roux-en-Y gastric bypass, biliopancreatic diversion). There is the potential for surgery which results in malabsorption to reduce the absorption of oral contraception, exacerbated by diarrhoea and/or vomiting.
As previously stated, UKMEC relates to the safety of the contraceptive rather than its efficacy and this should be borne in mind. The UKMEC 2016 states that there is limited evidence that oral contraceptive efficacy is unaffected by gastric bands and biliopancreatic diversions. However, it also states that other (pharmacokinetic) studies have shown conflicting results for the efficacy of oral contraception in women who have undergone a jejunoileal bypass. Therefore, until more research has been done, it would be prudent to discuss the potential for decreased efficacy of oral contraceptives with women who have had bariatric surgery.
Organ transplant
This is also a new section. If a woman has had an uncomplicated organ transplant, all methods, including CHC, are UKMEC 2. However, if the transplant is complicated, e.g. by graft failure or rejection, CHC and the initiation of a Cu-IUD and LNG-IUS are UKMEC 3.
Gestational trophoblastic disease
All non-intrauterine methods of contraception can be used at any point after gestational trophoblastic disease. The only concern is the use of intrauterine contraceptives when levels of beta-human chorionic gonadotropin (bHCG) hormone are still detectable. If there are persistently elevated levels of bHCG or malignant disease, intrauterine contraception is contraindicated (UKMEC 4), but if the levels of bHCG are decreasing, intrauterine contraception (Cu-IUD and LNG-IUS) are UKMEC 3.
Breast disease
If a woman develops a breast mass or breast symptoms while using CHC, she may continue with it (UKMEC 2), but if she had the symptoms before starting, CHC should be avoided (UKMEC 3).
Cervical cancer – radical trachelectomy
As the anatomy of the uterus will have altered due to the surgery, it is recommended that any intrauterine contraception is inserted in a specialist setting (UKMEC 3).
Asymptomatic chlamydia
Many women have chlamydia without exhibiting any symptoms or signs. If an asymptomatic woman is found to have chlamydia, the insertion of a Cu-IUD or LNG-IUS would be best delayed until she has been treated (UKMEC 3). However, if a woman who has asymptomatic chlamydia needs a Cu-IUD for emergency contraception, it can be inserted at the same time that treatment for chlamydia is initiated.
HIV infection
Generally, HIV infection does not prevent any contraceptive being used. However many antiretroviral medications can induce liver enzymes and therefore reduce the efficacy of most hormonal contraceptives. In view of the risk of infection, in women who have HIV infection and low CD4 counts (
Rheumatic diseases
This is a new addition to the UKMEC. Women who have rheumatoid arthritis can generally use all contraceptive methods (UKMEC 2) although the Cu-IUD is the only one that has unrestricted use (UKMEC 1).
In SLE, it is the presence or absence of antiphospholipid (aPL) antibodies which determines the suitability of most contraceptive methods. The Cu-IUD has unrestricted use (UKMEC 1) whether or not the woman has aPL antibodies. Other contraceptive methods are UKMEC 2 in women with SLE who do not have aPL antibodies. Any woman who has positive aPL antibodies (even if they do not have SLE) cannot use CHC (UKMEC 4) but all other contraceptives can generally be used (UKMEC 2). This is a change from previous UKMEC guidance where progestogen methods were UKMEC 3 in the presence of positive aPL.
SUMMARY
The UKMEC 2016 guidance provides up-to-date recommendations on the suitability of different contraceptive types for women with different medical conditions. The additional resources in the document to aid the correct diagnosis of migraine headaches, with or without aura, make the guidance an indispensable resource for all clinicians dealing with contraception. All clinicians should ensure they have easy access to this document during their clinics so that they can refer to it when needed.
TEST YOUR KNOWLEDGE
You can undertake a CPD module on this subject to assess your knowledge. Click here or select the module from the Curriculum menu.
If you have answered the self-assessment questions in the printed journal, complete the module to check your answers and to gain a certificate of completion to add to your revalidation portfolio.
REFERENCES
1. The Faculty of Sexual and Reproductive Healthcare FSRH 2016; UK Medical Eligibility Criteria for contraceptive use. UKMEC 2016. Available from http://www.fsrh.org/standards-and-guidance/documents/ukmec-2016/
Related articles
View all Articles