Advances in inhaler technology: influences on choice
Jane Scullion
Jane Scullion
BA(Hons), RGN, MSc
Consultant Respiratory Nurse
University Hospitals of Leicester,
Director of Education UK inhaler Group
Dr Omar Usmani
MBBS, PhD, FHEA, FRCP, FERS
Reader in Respiratory Medicine and Consultant Physician Imperial College London and Royal Brompton Hospital, Chair of the UK Inhaler Group
Practice Nurse 2021;51(05):28-32
Advances in inhaler design have greatly increased our choices when it comes choosing a suitable inhaler, but the design is not the only consideration. An understanding of the technology can help guide the choices we make in partnership with our patients
Although there is evidence of the use of inhalation dating back to the ancient Egyptians, over 3,500 years ago,1 The British Pharmacopoeia of 1867 was the first official formulary for inhalation medicines. Modern day inhalers, as we know them, date back to 1956 with the production of the pressurised metered dose inhaler (pMDI).2 Since then there has been an exponential growth in inhalers, and in the pharmaceutical industry producing them. Inhalers have greatly improved the quality of life of millions of people, in terms of both morbidity and mortality, and we need to consider the technological advances that assist us in our choice of inhaler.
Early asthma medication was delivered in a squeeze bulb nebulizer (Figure 1), a combination of a rubber pump and glass bulb. This was somewhat unwieldy, fragile and unreliable, delivering large particles which were unlikely to reach the target areas in the lungs. We now know that it is particles of less than 5 μm diameter that are carried into the lungs.3 Larger particles deposit in the bronchial/conducting airways and smaller particles (0.1-1 μm diameter) deposit in the peripheral airways.3
PRESSURISED METERED DOSE INHALERS
In 1956 Riker designed the first pressurised metered dose inhalers (pMDI), uniting two relatively new technologies; the chlorofluorocarbon (CFC) propellant producing the aerosol, and the Meshburg metering valve, originally developed to dispense perfume.4
A pMDI delivers a specified dose of the active agent or agents in each inhalation or puff.5 A puff consists of a suspension or dispersion of one or more active ingredient in a propellant, a mixture of propellants or a mixture of solvent and propellants. The role of propellant in a pMDI system is to provide the required pressure to atomize the drug formulation into micron-scaled droplets. The advantage of pMDIs is that they produce accurate and repeatable dosing.6
By 1956 Riker had developed two pMDI based products, the Medihaler-Epi which contained epinephrine (adrenaline), a non-selective adrenergic agonist, and the Medihaler-Iso containing isoprenaline, a non-selective beta-adrenergic agonist.2 Both these medications gave short term relief from asthma symptoms.2
Spacers
One of the issues with pMDIs is the need to co-ordinate actuation with inhalation, a problem often due to lack of correct instruction. This led to the development of the spacer, which largely overcame this issue.
A spacer adds a reservoir between the mouthpiece of the pMDI and the patient’s mouth. A valved holding chamber (VHC), a reservoir with a one-way valve permitting airflow into, but not out of the patient's mouth, was developed in the 1950s.7,8 The use of a spacer or a VHC means that aerosolised particles from the pMDI are slowed down and lung deposition improved.
Spacers are essential for children as they are unable to coordinate actuation and inhalation, and they are useful in all ages where coordination is an issue or where a person is assisted with their inhaler use. They are also recommended for emergency relief.9
Breath-actuated pMDIs
The problem with synchronisation of actuation and inhalation also led to the development of a breath-actuated pMDI. Riker developed the first breath-actuated pMDI, the Autohaler, in 1970;7 first the Duohaler (isoproterenol hydrochloride and phenylephrine bitartrate) and not long afterwards the Iso-Autohaler (isoproterenol).7
The Autohaler was a pocket-sized device, simple to use and convenient, but wasn’t very popular sales-wise.8 This may be because the original drug formulations were not particularly effective as asthma treatments and, by the time it contained either a beta agonist or inhaled corticosteroid, there were many alternatives that were more popular. The fact that the red lever snapped shut on inhalation also led some health care professionals to postulate that this would stop the inhalation prematurely.
Inspiratory flow and deposition
When a pMDI is used the inspiratory flow rate will determine the velocity of the airborne particles. The faster they travel the greater the probability of impaction in the oropharynx and larynx. A slow inhalation, a flow rate of about 30 litres per minute (l/min), is considered optimal.10 This means inhaling fully, after deep exhalation, over 2-3 seconds for a child and over 4-5 seconds for an adult.10 The older pMDIs have a low deposition: even with an optimal technique the actual amount of medication reaching the lungs is estimated to be around 12%.11
Technological advances have led to the development of a softer plume and, alongside changes in the valve within the inhaler and smaller particle sizes, deposition has increased to equal that of using a spacer device in some inhalers. Spacers, however, are still necessary for children and those with coordination problems.12
Chlorofluorocarbon propellants
Despite successes in asthma control and symptom relief in both asthma and COPD the chlorofluorocarbon (CFC) propellant in pMDIs and other aerosol products was found to be contributing to damage to the ozone layer and global warming. In 1987, the United Nations developed the Montreal Protocol, addressing the harmful effects of CFCs on the ozone layer. As a result, hydrofluoroalkanes (HFAs) gradually replaced CFCs as the propellant in pMDIs.7
Traditional, CFC-containing pMDIs had to be shaken to ensure that the aerosol released from the inhaler contained a uniform dose of medication, because the propellant CFC was in a solution, not a suspension.13 Many HFA formulations are in true solution, but not all, so shaking is not necessary for all pMDIs.14 However, as shaking unnecessarily does not have detrimental consequences, advice to shake the inhaler before use is seen as a necessary step to avoid confusion.15 The introduction of HFAs also minimised the ‘cold freon effect’, where patients would stop inhaling as a cold plume hit the airways.
The effect of pMDIs on global warming remains a topic that is hotly debated. HFAs also affect the environment. Lower global warming potential (GWP) propellants are currently in development. Of the current HFAs, HFA134a is more widely used but HFA227ea has a lower GWP so this technological advance may influence our choice. A person’s ability to use an inhaler and the total GWP of production and disposal of all inhalers need to be considered when we think about our choices.
SOFT MIST INHALER
The soft mist inhaler (SMI) is a development of the aerosol, presently only available as the Respimat. This offers a fine mist, multi-dose and propellant-free inhaler,16 that is now refillable, thus improving the GWP of inhalers by reducing the number sent to landfill. Its technological advances include a warning that the inhaler is running out of doses, and the cartridge containing the medication automatically unloads when empty and cannot be used.16
DRY POWDER INHALERS
In 1864 Alfred Newton patented an inhalation device for the delivery of dry powder medications – a dry powder inhaler (DPI).17 His device was based on the principles that persist to the present time, namely:
- The powder should be kept dry
- The drug should be finely ground and delivered as a fine powder.
Unfortunately Newton also believed that potassium chlorate would be beneficial to the lungs.18 In the 1940s, the Aerohalor device was launched containing penicillin G powder, and in the 1950s, this device was used for the delivery of isoprenaline.17,18 The Aerohalor is considered to have been the first commercially available DPI.18
DPIs enclose the actual medication micronized in a carrier which can be packaged in single dose quantities, in blisters or gel capsules, or as a powder formulation. Although inhalers containing just one medication will deliver accurate and repeatable dosing, when more than one medication is combined, care must be taken to ensure that particle sizes and the mix of medication delivers reliable doses.
All DPIs are characterised as low, medium or high resistance devices. During inhalation, the agglomerated particles must be dispersed, and this, to some extent, depends on the inspiratory flow rate generated by the person using the inhaler.19 Inhalation needs to be fast and deep from the start of inspiration, at a flow rate of around 60 l/min.19 Inspiratory flow rates also influence the amount of the dose that is released, with a higher variability at lower flow rates. The delivery of drug to the lung is dependent upon the person’s peak inhaled flow rate.19
We also need to consider the internal resistance of the device. This will affect the ability of an individual to achieve the required flow rate of 60 l/min and may be problematic in people with COPD or constricted airways.19
DPIs are breath actuated. As they eliminate the need to coordinate activation and inhalation the correct inhalation manoeuvre is not as complex. Unlike a pMDI the majority should not be shaken, the Easyhaler being one exception, and users should not exhale into the device as this creates moisture which can affect the powder.
CHOOSING AN INHALER
Whatever we prescribe, we cannot assume that the patient will use it correctly. People often make many mistakes and critical errors with all types of inhaler, even with repeated instruction. Dekhuijzen and his colleagues listed four principles, proposing that a better matching of patient with device could be accomplished if the prescriber was aware of:
1. The patient characteristics (disease, severity, fluctuation in airflow obstruction, etc.)
2. What class of medication is indicated
3. Where in the lung the medication should be delivered
4. How this can be best achieved by a given device in this specific patient.20
They also proposed considering the matching of the device and an evidence-based approach, enabling the prescriber to make a rational choice in only a few minutes by just considering the following four, simple questions:
- Who?
- What?
- Where? And
- How?
This was called the 3W-H approach and is a practical approach for prescribing inhalers.20
Whether we or our patients prefer a pMDI, DPI or SMI, what is important is the inspiratory effort needed to get the medication to where it is required and this is also affected by the internal resistance of the inhaler. Although there are low, medium and higher resistance DPIs, this information is often lacking in the product information.
In low-resistance DPIs, the disaggregation and the microdispersion of the drug is highly dependent on the patient’s airflow inhalation rate. This is because the role of the resistance-induced turbulence is not important in these inhalers. This doesn’t make the low resistance DPIs the best performing DPIs because their intrinsic low-resistance regimen means that the patient requires a higher inspiratory airflow rate and effort to activate them. This may not be possible for patients with airflow limitation. Examples here include the Aerolizer and the Breezhaler
In medium-resistance DPIs flow-rate dependency is less important as there is sufficient turbulence to disaggregate and disperse the medication even without a maximal inspiratory flow rate. Examples of medium resistance DPIs include Genuair, Novolizer, Turbohaler, Accuhaler and Ellipta devices. There needs to be a balance between the inhalation flow rate and the intrinsic resistance of the DPI in order for the particulate deposition of the inhaled dose to be optimal and, therefore, effective.
High resistance inhalers are more effective in particle generation and drug dispersion even with lower respiratory flow rates. Examples include the Easyhaler, Handyhaler and Twisthaler.21
There are also many devices that aim to help us determine either a person’s inspiratory flow, such as the Incheck device, or whether a person can inspire adequately for a particular inhaler, for example the Turbowhistle, the two-tone device, the Cliptone and the ‘AIMS’ machine. However, none of these will show us whether a person can effectively use the inhaler or co-ordinate inspiration and inhalation.
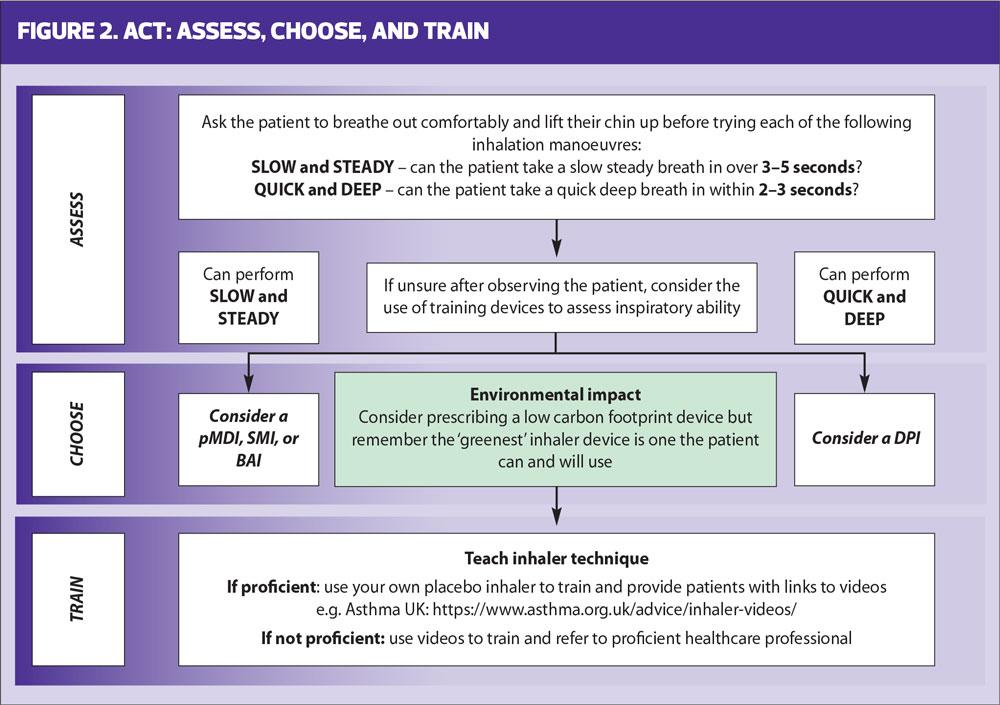
A pragmatic algorithm for assessing natural inspiratory flow was developed by Usmani and colleagues and updated in 2020 (Figure 2).22
SUMMARY
The plethora of devices available and technological advances should improve our choices and options, but it should also improve outcomes. Box 1 lists some of the factors that may influence our choices.23
Other factors that undoubtedly influence our choices are cost, the existence of local prescribing formularies and pressures to follow these formularies. While these factors are important they should not be the overriding considerations. The most expensive and least effective inhaler is the one that the patient cannot or will not use.
Despite the technological advances we have described here, a systematic review, covering 40 years and including 144 studies, demonstrated that we haven’t made any forward strides. The conclusion is that to improve morbidity and mortality in respiratory disease it has to be about more than the inhaler device.24
While technological advances have led to improvements in inhaler design, no single inhaler has overcome all the problems for each individual requiring and using inhalers.
REFERENCES
1. British Pharmacopoeia 1867, Published under the direction of the General Council of Medical Education and Registration of the United Kingdom, pursuant to the Medical act (1858). General Medical Council (GB), 1880
2. Purewal Tol S, Grant DJW. Metered Dose Inhaler Technology (Illustrated ed.). Informa Health Care 1997. ISBN 1-57491-065-5.
3. Swarbrick J. Encyclopedia of Pharmaceutical Technology (3rd Illustrated ed.). Informa Health Care 2007. ISBN 0-8493-9394-9.
4. Clark A. Medical Aerosol Inhalers: Past, Present, and Future. Aerosol Sci Technol 1995;22(4):374–391.
5. Hindle M, Newton DA, Chrystyn H. Dry powder inhalers are bio equivalent to metered dose inhalers A study using a new urinary albuterol (salbutamol) assay technique. Chest 1995;107(3):629-33
6. Roche N, Dekhuijzen PNR, The evolution of pressurized metered dose inhalers from early to modern devices J Aerosol Med Pulm Drug Deliv 2016;29(4):311-27
7. Franklin W, Lowell FC, Michelson AL, et al. Aerosolized steroids in bronchial asthma. J Allergy 1958;29:214–221.
8. Stein SW, Thiel CG. The history of therapeutic aerosols: a chronological review. J Aerosol Med Pulm Drug Deliv 2016;30:20–41
9. Vincken W, Levy M, Scullion J, et al. Spacer devices for inhaled therapy: why use them, and how? On behalf of the ADMIT group. ERJ Open Research 2018;4:00065-2018; DOI: 10.1183/23120541.00065-2018
10. Laube BL, Janssens HM, de Jongh FHC, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J 2011 37;1308-1417
11. Lavorini F, Magnan A, Dubus JC et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med 2008;102(4):593-604
12. Levy ML, Dekhuijzen PNR, Barnes PJ, et al. Inhaler technique: facts and fantasies. A view from the Aerosol Drug Management Improvement Team (ADMIT). NPJ Prim Care Respir Med 2016;26:16017 doi: 10.1038/npjpcrm.2016.17
13. Hendeles L, Colice G L, Meyer RJ. Withdrawal of albuterol inhalers containing chlorofluorocarbon propellants. N Engl J Med 2007;356(13):1344–1351 doi: 10.1056/NEJMra050380
14. Buttini F, Miozzi M, Balcucci AG, et al. Differences in physical chemistry and dissolution rate of solid particle aerosols from solution pressurised inhalers. Int J Pharm 2014;465:42–51
15. Usmani OS, Scullion J, Keeley D. Our planet or our patients - is the sky the limit for inhaler choice? Lancet Respir Med 2019;7(1):11-13
16. Scullion J. Are all inhalers the same? Let’s Talk Respiratory 2019 https://letstalkrespiratory.com/are-all-inhalers-the-same/
17. Anderson P. History of Aerosol Therapy: Liquid Nebulization to MDIs to DPIs. Respir Care 2005;50(9):1139–1150.
18. Sanders M. Inhalation therapy: an historical review. Prim Care Respir J 2007;16(2): 71–81.
19. Mahler DA. Peak inspiratory flow rate as a criterion for dry powder inhaler use in chronic obstructive pulmonary disease. Annals Am Thorac Soc 2017;14(7):1103-1107
20. Dekhuijzen PNR, Vincken W, Virchow JC, et al. Prescription of inhalers in asthma and COPD: towards a rational, rapid and effective approach. Respir Med 2013;107(12):1817–1821.
21. Dal Negro RW. Dry powder inhalers and the right things to remember: a concept review. Multidiscip Respir Med 2015;10(1):13 doi: 10.1186/s40248-015-0012-5. eCollection 2015
22. Usmani O, Capstick T, Saleem A, et al. Choosing and appropriate inhaler device for the treatment of adults with asthma or COPD. 2020. https://www.guidelines.co.uk/respiratory/inhaler-choice-guideline/455503.article
23. Scullion J. Striking a balance: device choice and therapeutic dosages. Guidelines for Nurses. 2019. https://www.guidelines.co.uk/expert-articles/striking-a-balance-device-choice-and-therapeutic-dosages/455377.article
24. Finlay WH. The mechanics of inhaled pharmaceutical aerosols: an introduction. Academic Press, 2001.
Related articles
View all Articles