An inspector calls: Medicines management
The author is a professional adviser who regularly participates in Care Quality Commission inspectio...
The author is a professional adviser who regularly participates in Care Quality Commission inspections in general practice
As practice nurses, we may doubt that CQC inspections have much to do with ‘us’ – but one of the areas inspectors check is medicines management – which means that our vaccine storage and stock control will be scrutinised
The Care Quality Commission (CQC) is in the process of revising its inspection processes but in the meantime, inspections in general practice continue and one of the areas of scrutiny is the management of medicines (Outcome 9, Regulation 13).
With this outcome the CQC will be looking to see whether the registered GP has suitable arrangements in place to ensure people have their medicines when they need them, and in a safe way, and also that people are given information about their medicines.
The Inspector will make a judgement on whether the practice is compliant or non-compliant with this outcome, usually by gathering and assessing information from different sources.1 These may include reviewing policies and procedures, observing practice, talking to patients and staff and checking the process for ordering, receiving, storing, dispensing and disposing of medicines.
Some 23% of practices inspected were found to be non-compliant against at least one essential standard.2
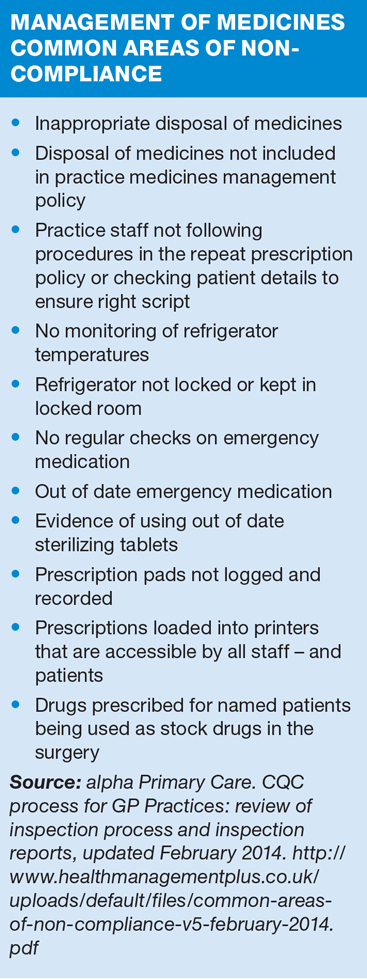
It is the GP’s responsibility to have a robust medicines management policy in place, which clearly defines the way in which medicines are stored, handled, transported, and administered and practice nurses are often involved – particularly with their role in administering vaccines. Much of the identified non-compliance has been around refrigerator management and maintenance of the cold chain.
There should be reliable procedures in place to ensure that medicines are given to the right person at the right time, such as a repeat prescribing policy that includes conducting medication reviews.
The CQC will also expect patients to be provided with information about their medication, and to be aware of their own treatment. Practices should also ensure that all staff involved with the handling or prescribing of medicines are appropriately trained and can provide evidence of this training. In addition, practices should also have policies in place to minimise risk, and can show that they record, investigate and share concerns where adverse, medicines-related, events occur, either in the form of mishandling, allergies, or near misses. There should also be a procedure for disseminating and acting on local/national clinical guidance, Medicines and Healthcare products Regulatory Agency (MHRA) alerts, national and local formularies and patient safety alerts to staff.
EXAMPLES OF NON-COMPLIANCE
A recent inspection found that prescription-only oral, rectal and injectable medicines were stored in unlocked cupboards. Furthermore, there was no clear rationale why some of these medicines were being kept. There were no arrangements in place to record either receipt of medicines, or when, or to whom they had been administered. The inspectors saw some injectable medicines that were left on a trolley in one of the surgeries. The doctor confirmed they were kept there permanently. This meant that some medicines were not stored safely and their use was inadequately recorded.
Inspectors looked at the vaccination and other medication storage arrangements at one practice, and records of administration of medication. They noted that most of the medication storage was secure and suitable. However, some of the injections were stored in an unlocked cupboard in a consultation room. Vaccinations were stored in an unsecured refrigerator. There were no up to date records of regular checks of medicines in stock to ensure that they were used before expiry. The provider told the inspector that this used to be managed by a nurse who was on long term leave at the time of the inspection. They found a batch of expired flu vaccine for children in the refrigerator. There was another batch of hepatitis A and B vaccine for children that had also expired. They also found seven expired packs of contraceptive injections stored in one of the consultation rooms. This meant that patients were at risk of having out of date medication administered to them. No records were available of any recent medication audits for the clinic.
At another practice inspectors saw that the medicines used for anaphylactic reaction, for example following a vaccination, were not kept in the area where they might be needed. This meant that medicines that may have been required rapidly were not immediately available.
Inspectors asked staff whether there was an emergency drug kit in the practice and were told there were no emergency drugs available. Following the inspection they were informed that emergency drugs were stored in the nurse’s room in a large box that was ‘prominently’ labelled. However, staff with whom inspectors spoke were unaware of it. One member of staff said that a request had been made to the senior GP to arrange for a supply of emergency drugs to be made available for clinical staff at the practice. In this practice, there was a real risk that staff would be unable to provide treatment to people in an emergency as they were unaware of where emergency drugs were located.
At the same practice, medicines were not kept safely. Inspectors looked in two refrigerators in two clinical rooms, and at the records of the respective daily temperature recordings. They found that on one record, refrigerator temperature recordings had not been completed in the last two days, which is not in line with published best practice guidance.
Medicines were not disposed of appropriately. There was a store of expired medication held in the two refrigerators. Inspectors were told that there were arrangements in place for the disposal of the medication. When asked for records of such arrangements, however, they were told that there was no specific date arranged. This meant that medicines were not disposed of in accordance with instructions.
Inspectors observed that the refrigerator in which vaccinations and medications were stored had a digital thermometer, but there were no records to demonstrate that the alarm had been regularly tested to ensure that it was functioning properly. They noted that daily upper and lower temperature checks of the refrigerator to ensure the refrigerator temperature had not gone out of range were not recorded. This meant that the provider could not be assured of cold chain storage validity of vaccinations and medications were stored in a way that could put people at risk.
The practice manager told inspectors that the content of the refrigerator was regularly checked so that any expired vaccinations or medications could be disposed of, and re-ordered if required. However, there were no records available to demonstrate when the checks had been conducted. At the time of the visit inspectors observed that two vaccinations had expired. These were immediately removed for safe disposal. They noted that there was no clear system to rotate the content of the refrigerator according to the stated expiry dates.
There were shortfalls in medicines management because the provider had not ensured there were appropriate arrangements in place for the storage, recording and disposal of medicines. The refrigerator in the nurse’s room had a temperature chart. However, for a period of 10 days the temperature had not been recorded. According to the record, the thermometer (integral to the refrigerator) was never reset, while an additional thermometer inside the refrigerator did not work. The signing off for checking the refrigerator was not always done; this refrigerator also contained flu vaccines that had expired.
REFERENCES
1. Care Quality Commission. How we inspect GPs and primary medical services when they are registered. http://www.cqc.org.uk/content/how-we-inspect-gps-and-primary-medical-services-when-they%E2%80%99re-registered
2. PRWeb. ‘23% of GP practices found to be non-compliant.’ Press release, available at: http://uk.prweb.com/releases/2013/8/prweb11048542.htm
Related articles
View all Articles