Potential implications of monoclonal antibodies in primary care
Mariana Carreno, Seen-Wai Lavelle
Mariana Carreno, Seen-Wai Lavelle
Medical, Sanofi, Reading, UK
Practice Nurse 2023;53(4):20-27
Although monoclonal antibodies (mAbs) are classically reserved for initiation and management in secondary care, some mAbs are now administered in primary care, with promising further expansion in this setting
Monoclonal antibodies (mAbs) are a highly specific clinical tool used for the management of a wide range of ailments, including cancer, dermatology and cardiovascular conditions, and infectious diseases. The field of mAb use has risen exponentially in recent years, particularly during the COVID-19 pandemic, and the development of new mAbs is becoming one of the fastest-growing segments of biomedical research. As such, mAbs are expected to help tackle major global health threats in the future, most notably future pandemics and antimicrobial resistance.
Given the increase in mAb approvals and applications in clinical practice, along with their growing relevance to the primary healthcare professional, this article aims to review their conception and advancements, and their potential impact in primary care practice.
BACKGROUND
The immune system exists to protect against disease through a complex interplay between two main subsystems: innate and adaptive.1 The former acts as an initial line of defence and is characterised by a rapid, broad-acting response.1 The latter comprises a response highly specific to the intruding pathogen, which, although slower to mount on initial exposure, can be preserved through immune memory for a faster, more efficient response upon subsequent encounters.1 B cells, an adaptive component, can recognise specific regions of foreign material, known as antigens, and with the help of innate cell types and T cells, can produce antibodies which recognise and bind to specific regions of that antigen.1,2 Once bound, antibodies act to aid in the destruction and removal of such material.1,2
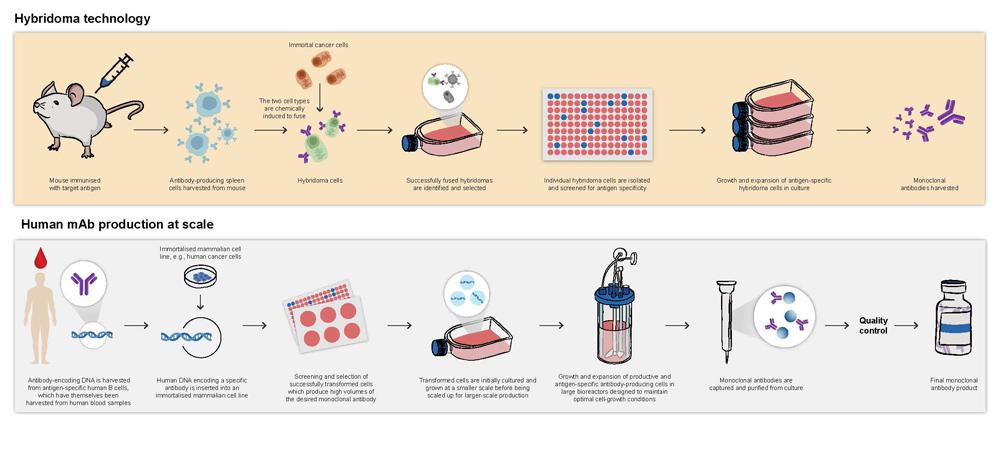
Antibodies produced during a typical immune response are polyclonal with a variety of different B cells producing a range of antibodies of differing specificities.2,3 However, monoclonal antibodies are designed, and laboratory produced, through various technologies to be highly specific for a single target molecule.2,3 This precision can be used to target disease-associated antigens and effectively combat infection and disease progression.4 The first mAb was generated via the hybridoma approach in 1975. (Visit practicenurse.co.uk to view an illustration of this process.) Since then, techniques have evolved to allow mass production of antibodies that are optimised for clinical purposes (Figure 1).3,5
Early mAbs were entirely animal in origin and limited in their clinical application due to the human immune system recognising them as foreign material, resulting in the potential for both hypersensitivity reactions and relatively quick clearance of the mAbs from the body.2,6,7 Modern techniques have overcome these issues by increasing the human component of mAbs, including the production of fully human mAbs.2,6,8 These techniques include genetic modification of animal-derived mAbs to express components of human antibodies, the use of transgenic animals (animals genetically modified to express genes encoding human antibodies) and recombinant DNA technologies which modify various cell lines to produce fully human mAbs.2,9
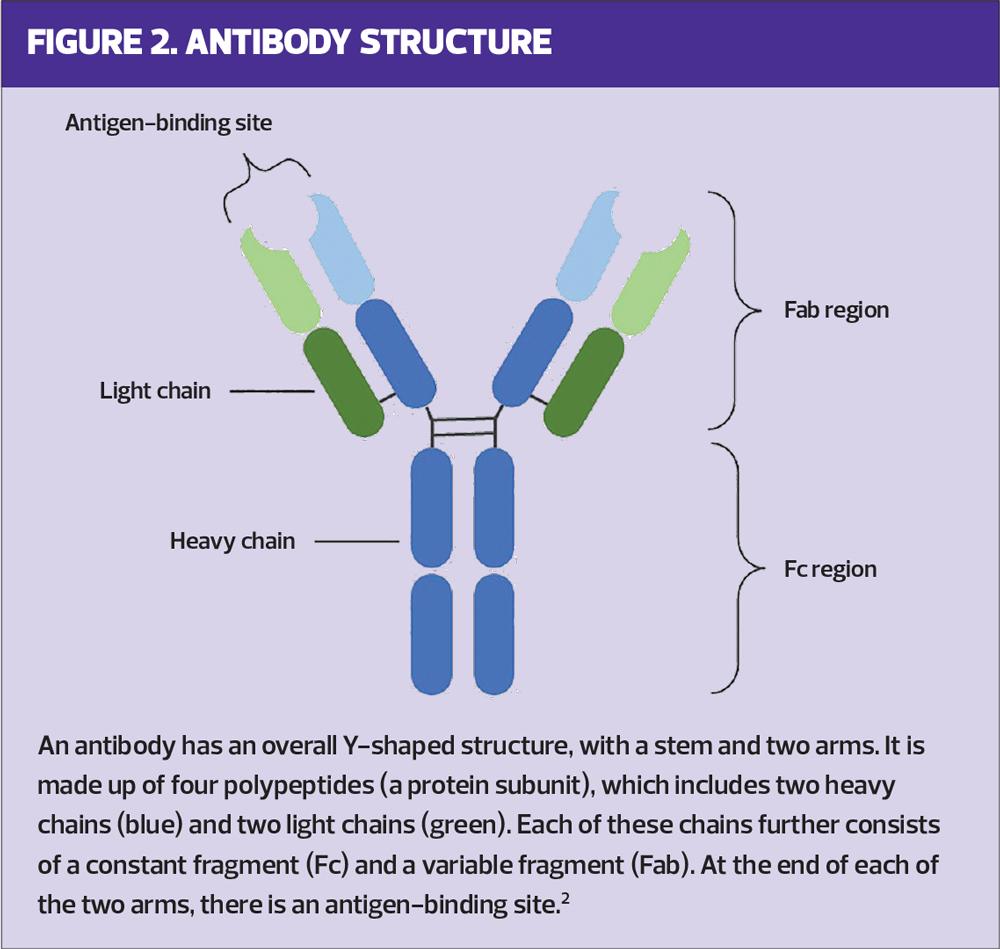
ANTIBODY STRUCTURE AND FUNCTION
Antibodies possess a unique structure which lends itself to their functional roles.2 Their general structure can be described as Y-shaped and is composed of two functional regions: the constant fragment (Fc) and the antigen-binding fragment (Fab) (Figure 2).2,3 The Fab region binds to the target antigen, while the Fc region mediates the antibodies’ effector functions within the immune response.2,4 mAbs harness these capabilities, undergoing an extensive screening process to ensure highly specific and effective mAbs are selected for their intended use.10-12 Antibodies can be divided into five classes based on structural differences: immunoglobulin (Ig) A, IgD, IgE, IgG and IgM, with IgG being the most frequently used for developing mAbs due to its ability to elicit appropriate effector functions and its longer serum half-life compared with other antibody classes.2,12
mAbs can exert their effects directly or indirectly.2,13 They can provide direct protection by binding to their target (via their Fab region), rendering the bound target harmless, and subsequently tagging it for destruction.2,13 In the case of infectious diseases, this neutralisation can prevent pathogen entry into the cell, and in the case of noncommunicable diseases, this can block interactions between signalling molecules and receptors in a disease-causing pathway.2,13 Indirect mechanisms (i.e., effector functions) rely on antibody Fc region interactions with other aspects of the immune system after antigen binding.2,13 Other immune cells can bind to the Fc region via specialised Fc receptors, or the Fc region can interact with a group of serum proteins known as the complement system.2,13
PROPHYLACTIC mAbs FOR INFECTIOUS DISEASE PREVENTION
mAbs can function as both a prophylactic measure to prevent infection and a post-infection treatment option to prevent the development, and aid in the resolution, of disease.16 For effective prophylaxis, different mAbs must confer different levels of immunity depending on their target pathogen.17 Sterilising immunity, where the pathogen is eliminated before it can infect or replicate in the host, is necessary, for example, in human immunodeficiency virus, where initial exposure can result in the virus integrating into human cells and evading any subsequent immune response.18-20 In other circumstances, a mAb might not provide sterilising immunity but may still be beneficial in protecting against severe disease, and allow sufficient antigen to circulate to allow a typical immune response to develop.17
mAbs VERSUS VACCINES
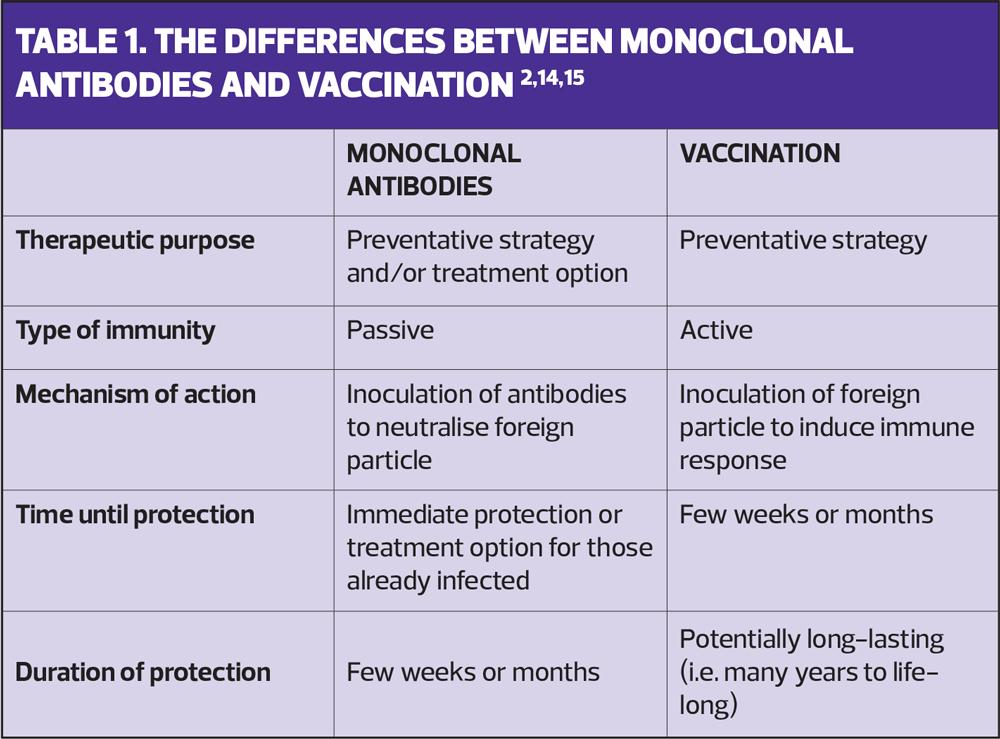
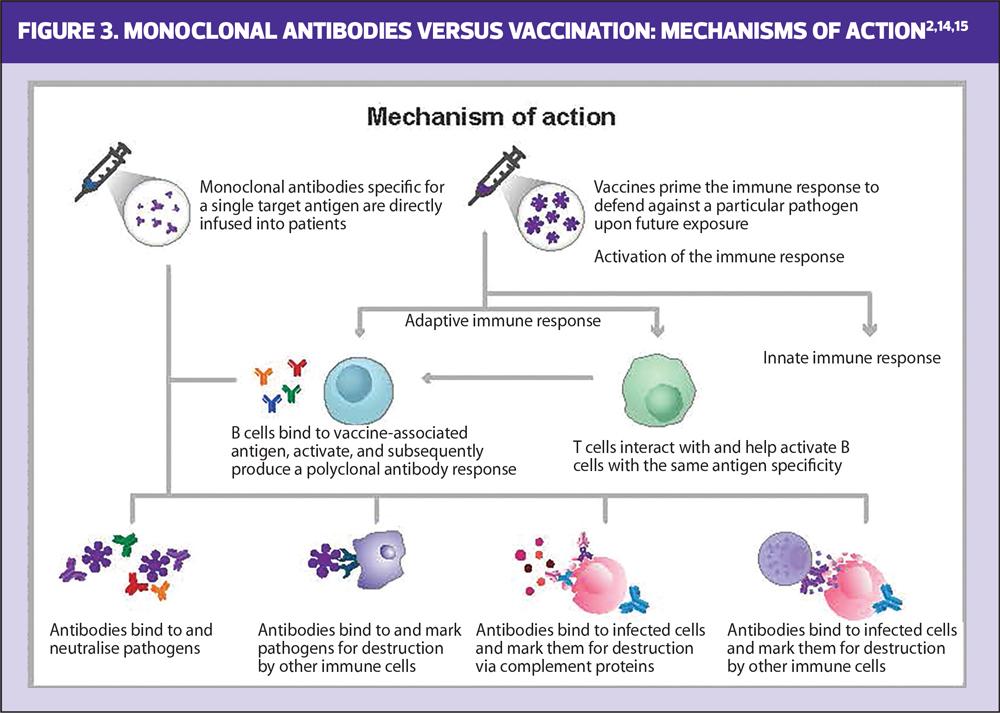
Infectious disease prevention has historically relied on vaccination, though mAbs have also been shown to effectively prevent infection when given prophylactically or immediately following exposure.16,21,22 While both vaccines and mAbs can potentially be used to prevent infections, their mechanisms of action differ (Figure 3).14 Vaccines deliver an antigen, which generates an active immune response, priming it to defend against future exposure to the pathogen.14,15 This memory response can be short (e.g. one influenza season) or long (e.g. life-long protection from measles), may require multiple doses of antigen, and typically takes ~2 weeks to develop after immunisation.14,15,23 In contrast, mAbs provide rapid passive immunity through direct introduction of disease-specific antibodies, making them an effective strategy for immunocompromised individuals or those unable to receive a vaccine (Table 1).14,24,25
As vaccines activate the human immune response, a variety of pathogen-associated antigens can be selected and included within the vaccine to induce a polyclonal response, where a range of antibodies specific for different regions of varying antigens are produced.2,3,14 Some vaccines are also able to induce T-cell-mediated cellular immunity.2,26 By contrast, mAbs are uniform in their specificity for a given region of a single antigen (known as epitope, and selected due to its critical involvement in the pathogen’s ability to cause infection) and do not generate a humoral or cellular immune response.2,27
As the immune mechanisms differ between vaccines and mAbs, and due to the high target specificity of mAbs, immune interference between the two preventative measures is unlikely if their target antigen and/or pathogen differs.28 This means that in most cases, mAbs can be safely and effectively administered alongside scheduled vaccines.28
mAb USE IN CLINICAL PRACTICE
The first therapeutic mAb was approved in both Europe and the US in 1986 for prevention of acute transplant rejection.29 Since then, mAbs have been developed as a clinical tool in a variety of conditions, such as cancer, infectious diseases and autoimmune diseases.30-32 More recently, mAb use has grown exponentially, with more than 500 mAbs in clinical development in 2020, and the Food and Drug Administration licensing the 100th mAb in 2021.9,33 Despite the continued clinical advancement of mAbs, there is an ongoing need to increase awareness, acceptance and accessibility among healthcare professionals (HCPs), patients and the general public.34-36 To fully address these issues, it is important that HCPs, including those in primary care, are well informed about the availability, applicability, and benefits of mAb immunotherapy.
mAbs IN UK PRIMARY CARE
Initiation of mAbs has traditionally been undertaken by specialists in secondary care, only to be continued in primary care after the first few doses have been monitored for adverse events (AEs).37 However, potential initiation in primary care could lead to improved accessibility and simplification of patient pathways, as well as improved therapy of conditions typically managed in primary care settings.38,39
Covid-19
Recent studies have found sotrovimab and tixagevimab/cilgavimab to be not only effective at preventing severe COVID-19, but also manageable to administer in a primary care setting.38,40,41 As primary care facilities usually act as a ‘first point of contact’ for members of the community, and as primary HCPs are uniquely positioned to identify those most at risk of severe infection, mAbs could be used as an early tool to prevent disease progression and reduce risk of hospitalisation.38,42-44
Hyperlipidaemia
Proprotein convertase subtilisin/kexin type 9 (PCSK9) mAbs in individuals requiring lipid-lowering beyond the capabilities of statins have become a valuable addition to hyperlipidaemia management in primary care.13 PCSK9 mAbs are initiated by a specialist, but because lipid management usually takes place in primary care, recipients often shift from a secondary to a primary care setting after a couple of years of effective immunotherapy.45 Although some mAbs require intravenous administration, PCSK9 mAbs can be given via self-administered subcutaneous injection, with prolonged intervals between doses, which could potentially promote therapy acceptance and adherence in recipients, thus making primary care-based management a viable option.46-48
Migraine
Fremanezumab has recently been approved in the UK as a preventative option for migraines in instances where previous preventative measures have failed.49 Although immunotherapy initiation is currently restricted to specialist HCPs, migraines are most commonly diagnosed and managed in primary care.50-52 Fremanezumab has shown to be effective and well tolerated; meanwhile, alternative treatments currently available to primary HCPs have demonstrated relatively low efficacy, with a host of side effects which often lead to discontinuation, suggesting a potential benefit of mAb implementation into routine primary care.53-55
Asthma
A steady increase in the number of approved and available mAbs for asthma, another condition often managed by primary HCPs, has been observed.29,56 Omalizumab and dupilumab, are two examples of mAbs currently approved in the UK as add on immunotherapies in cases of severe asthma where standard treatment has failed.57,58 Although prescription of these mAbs remains the responsibility of the specialist, primary care is still considered an essential element in effective disease management.56 The primary HCP’s role in this ‘shared-care’ system includes identifying individuals for specialist referral, monitoring recipients following mAb initiation via annual asthma reviews, and finally, if the recipient doesn’t experience any AEs after their initial mAb injections, taking over responsibilities for subsequent administration.37,56 Increased levels of relational continuity of care, that is the ongoing relationship between patient and HCP, in asthma management, a quality often associated with primary HCPs, has been linked to better health outcomes.59 Therefore, a holistic approach to asthma management in primary care may be beneficial.
Psoriasis
Management of psoriasis is dependent on disease severity, with mild-to-moderate cases most commonly being managed in primary care with first-line topical treatments, and moderate-to-severe cases requiring referral to specialist facilities for the initiation of second-line systemic treatment.60 mAbs, which include adalimumab, etanercept and ustekinumab, are considered a third-line therapy option, often reserved for individuals refractive to other systemic treatments.60,61 Unfortunately, psoriasis is often undertreated, with many individuals unable to achieve their treatment goals and subsequently voicing dissatisfaction with their current level of care.62,63 This presents a case for improved mAb access as they have demonstrated high efficacy as well as being reported to provide higher satisfaction rates than other systemic and topical therapies.64-66 Due to the lifelong nature of psoriasis, a primary healthcare setting could provide an easily accessible and efficient route for optimised long-term disease management that incorporates mAbs.67
Atopic dermatitis
Atopic dermatitis (AD) is generally managed in a primary care setting, with 97% of AD cases across the UK handled in general practice.68 First-line topical treatments for mild-to-moderate cases are commonly prescribed in primary care, with severe cases receiving specialist referral for prescription of systemic treatments including mAbs.69 Two mAbs are currently approved by the European Medicines Agency (EMA) for moderate-to-severe AD, dupilumab and tralokinumab, both of which show efficacy in reducing AD symptoms and improving quality of life.70,71 AD is a lifelong condition requiring substantial self-management; as such, primary HCPs are often relied upon to provide education and support.72 Implementation of mAbs into the primary care setting could help simplify a complicated disease-management regime, while simultaneously reducing the burden of multiple hospital visits.72,73
Osteoporosis
Denosumab is currently licensed in the UK for use in the prevention of osteoporotic fractures, especially for postmenopausal women.74 Upon licensing, due to a lack of specific safety concerns, it was decided that denosumab would be initiated in secondary care, but subsequent administration could occur almost exclusively in primary care settings.74 In a retrospective cohort study evaluating the clinical outcomes of individuals with osteoporosis treated with denosumab, the majority of patients transitioned to primary care-based administration following tertiary care initiation.39 This may indicate that as the public become more familiar with mAbs, the use of denosumab in primary care may grow.39
RISKS AND TOLERABILITY OF mAbs
Like all drug classes, mAbs can be associated with side effects following administration.75 Generally, mAbs are well tolerated, with commonly associated side effects including local skin reactions at the injection site and infusion-related reactions.75 Understanding the risks associated with each mAb can help HCPs monitor for, recognise, and promptly manage reactions.76 As mAbs possess characteristics that are different to many other drugs, mAb-associated AEs are distinct; therefore, a tailored AE classification system was developed in 2006 (Table 2).77
Unlike small-molecule drugs, which are primarily metabolised by cytochrome P450 enzymes in the liver, mAbs are broken down by circulating cells into amino acids, making mAb–drug interactions unlikely.78 Due to the global rise of polypharmacy, this characteristic is particularly advantageous.79
RECENT ADVANCES IN mAb TECHNOLOGY
mAb technology continues to advance, with researchers investigating potential improvements in mAb function and application.33 Until recently, mAbs targeted only one specific antigen; meaning complex, multifactorial diseases, such as cancer, that benefit from multiple therapy targets, required combination mAb immunotherapy.81 Bispecific antibodies, which are designed to bind two different antigens or epitopes, provide a broader immunotherapy option.81 Blinatumomab, a mAb indicated for the treatment of certain acute lymphoblastic leukaemia exemplifies the potential of future bispecific mAbs.81,82
Another improvement is the extension of mAb half-life by modification of the Fc region.21 This prolongs mAb duration after administration, reducing the number of doses required over any set period and promoting adherence.21,83 These long-acting mAbs have already been adopted for prophylaxis against infectious diseases.21,32,84 A single dose of nirsevimab has been shown to provide at least 5 months’ protection against medically attended respiratory syncytial virus lower respiratory tract disease, while tixagevimab/cilgavimab, for COVID-19 prophylaxis and treatment, has an extended half-life of around 3 months.21,32,84 The latter therapy suggests that mAbs can be an effective means of responding to emerging infectious pathogens, and possible future pandemics.85
COMBATING ANTIMICROBIAL RESISTANCE
Antimicrobial resistance (AMR) is a significant global public health threat, associated with an estimated 5 million deaths worldwide in 2019.86 The growing number of infections resistant to antibiotic treatment, along with the scarcity of innovation in the field of antimicrobials, has led the World Health Organization (WHO) to deem AMR both a major threat to humanity and a priority for innovative research.87 To address this issue, mAbs that target bacterial pathogens have been under development.22,88 This includes bezlotoxumab, a mAb approved by the EMA for effective prevention of recurrent Clostridium difficile infection.89 This is important as C. difficile is a common hospital-acquired infection, treated with antibiotics and associated with poor patient outcomes.22,90 Furthermore, mAb treatment of Staphylococcus aureus, a common cause of pneumonia, has shown great potential in preclinical studies.88,91
LIMITATIONS OF mAbs
It is evident that mAbs have many clinical benefits, but limitations remain. Although there is a shift to newer mAbs being fully humanised, certain available mAbs still retain some animal composition and thus, are more prone to eliciting anti-drug responses than entirely humanised mAbs.29,92 Furthermore, while some mAbs may require only a single injection, most administration schedules range from every two weeks to every two months.32,49,57,93 Although this schedule could still be considered beneficial compared with other drugs, repeated injections and/or infusions may be discouraging to recipients, potentially reducing adherence to treatment.14,48,94
CONCLUSION
mAbs are now an established clinical tool for a wide variety of indications, many of which are chiefly managed within primary care. Recent years have seen extensive innovation in their development, with future improvements predicted to help prevent major global health threats such as AMR, emerging pathogens and pandemics. Although mAbs have been typically administered by specialist HCPs, mAb use in primary care settings could be beneficial and manageable for both the recipient and HCP. Potential benefits include ease of access, increased continuity of care, avoidance of referral delays and poor primary–secondary care communication, and a welcome expansion to the primary HCP’s toolbox. However, for further deployment of mAbs in primary care settings, primary HCPs must be familiar with their safety and efficacy profiles and confident in administering them in their practice. Improved HCP awareness and demonstration of effective use in real-world primary care settings will be critical components in increasing access to mAbs for individuals.
REFERENCES
1. Marshall JS, Warrington R, Watson W, Kim HL. An introduction to immunology and immunopathology. Allergy, Asthma & Clinical Immunology. 2018;14(2):49.
2. Murphy K, Weaver C. Janeway's Immunobiology. New York: Garland Science, Taylor & Francis Group, LLC; 2017.
3. Levinson W. Review of medical microbiology and immunology. United States: McGraw-Hill Medical; 2008.
4. Malik B, Ghatol A. Understanding How Monoclonal Antibodies Work. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
5. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495-7.
6. Buss NA, Henderson SJ, McFarlane M, Shenton JM, de Haan L. Monoclonal antibody therapeutics: history and future. Curr Opin Pharmacol. 2012;12(5):615-22.
7. Niaudet P, Jean G, Broyer M, Chatenoud L. Anti-OKT3 response following prophylactic treatment in paediatric kidney transplant recipients. Pediatr Nephrol. 1993;7(3):263-7.
8. Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol. 2001;13(12):1551-9.
9. Lu RM, Hwang YC, Liu IJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27(1):1.
10. Li F, Vijayasankaran N, Shen AY, et al. Cell culture processes for monoclonal antibody production. MAbs. 2010;2(5):466-79.
11. Hanack K, Messerschmidt K, Listek M. Antibodies and Selection of Monoclonal Antibodies. Adv Exp Med Biol. 2016;917:11-22.
12. Salfeld JG. Isotype selection in antibody engineering. Nat Biotechnol. 2007;25(12):1369-72.
13. Blom DJ, Dent R, Castro RC, Toth PP. PCSK9 inhibition in the management of hyperlipidemia: focus on evolocumab. Vasc Health Risk Manag. 2016;12:185-97.
14. Centers for Disease Control and Prevention. Immunity Types c2021. https://www.cdc.gov/vaccines/vac-gen/immunity-types.htm.
15. Centers for Disease Control and Prevention. Understanding How Vaccines Work c2022. https://www.cdc.gov/vaccines/hcp/conversations/understanding-vacc-work.html.
16. Crowe JE, Jr. Human Antibodies for Viral Infections. Annu Rev Immunol. 2022;40:349-86.
17. Corti D, Purcell LA, Snell G, Veesler D. Tackling COVID-19 with neutralizing monoclonal antibodies. Cell. 2021;184(12):3086-108.
18. Nishimura Y, Igarashi T, Haigwood N, et al. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J Virol. 2002;76(5):2123-30.
19. Dashti A, DeVico AL, Lewis GK, Sajadi MM. Broadly Neutralizing Antibodies against HIV: Back to Blood. Trends Mol Med. 2019;25(3):228-40.
20. Wahl I, Wardemann H. Sterilizing immunity: Understanding COVID-19. Immunity. 2022;55(12):2231-5.
21. Griffin MP, Yuan Y, Takas T, et al. Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants. N Engl J Med. 2020;383(5):415-25.
22. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N Engl J Med. 2017;376(4):305-17.
23. Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nature Reviews Immunology. 2021;21(2):83-100.
24. Wise J. Covid-19: Evusheld is approved in UK for prophylaxis in immunocompromised people. BMJ. 2022;376:o722.
25. World Health Organization. Monoclonal Antibodies (mAbs) for Infectious Diseases. https://www.who.int/teams/immunization-vaccines-and-biologicals/product-and-delivery-research/monoclonal-antibodies-(mabs)-for-infectious-diseases.
26. Ura T, Takeuchi M, Kawagoe T, et al. Current Vaccine Platforms in Enhancing T-Cell Response. Vaccines (Basel). 2022;10(8).
27. Taylor PC, Adams AC, Hufford MM, et al. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol. 2021;21(6):382-93.
28. Esposito S, Abu-Raya B, Bonanni P, et al. Coadministration of Anti-Viral Monoclonal Antibodies With Routine Pediatric Vaccines and Implications for Nirsevimab Use: A White Paper. Front Immunol. 2021;12:708939.
29. The Antibody Society. Antibody therapeutics approved or in regulatory review in the EU or US c2022. https://www.antibodysociety.org/resources/approved-antibodies/.
30. Roche Diagnostics GmbH. Herceptin 150 mg powder for concentrate for solution for infusion SmPC c2010. https://www.ema.europa.eu/en/documents/product-information/herceptin-epar-product-information_en.pdf.
31. Roche Registration GmbH. MabThera 100 mg and 500 mg concentrate for solution for infusion SmPC c2008. https://www.ema.europa.eu/en/documents/product-information/mabthera-epar-product-information_en.pdf.
32. European Medicines Agency. Beyfortus: Summary of Product Characteristics c2022. https://www.ema.europa.eu/en/documents/product-information/beyfortus-epar-product-information_en.pdf.
33. Mullard A. FDA approves 100th monoclonal antibody product. Nat Rev Drug Discov. 2021;20(7):491-5.
34. IAVI/Wellcome. Expanding access to monoclonal antibody-based products: A global call to action c2020. https://wellcome.org/sites/default/files/expanding-access-to-monoclonal-antibody-based-products.pdf.
35. Kwan BM, Sobczak C, Gorman C, et al. "All of the things to everyone everywhere": A mixed methods analysis of community perspectives on equitable access to monoclonal antibody treatment for COVID-19. PLoS One. 2022;17(11):e0274043.
36. Rader B, Whaley CM, Rogers WS, Brownstein PJS, Cantor J. Assessment of geographic access to monoclonal antibodies in the United States. J Travel Med. 2022;29(3).
37. Centre of Excellence in Severe Asthma. Initiation of Monoclonal Antibodies for Severe Asthma in Primary Care c2019 updated 17 January 2019. https://www.severeasthma.org.au/initiation-in-primary-care/.
38. Böbel M, Joos S, Förster C. [Neutralizing monoclonal antibodies in COVID-19: a case series in general practice]. Dtsch Med Wochenschr. 2022;147(9):558-63.
39. Moran CP, English S, Beringer T, Lindsay JR. Real World Experience of Denosumab Treatment in the Belfast Osteoporosis Service. Ulster Med J. 2019;88(3):150-6.
40. Montgomery H, Hobbs FDR, Padilla F, Arbetter D, Templeton A, Seegobin S, et al. Efficacy and safety of intramuscular administration of tixagevimab–cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. The Lancet Respiratory Medicine. 2022;10(10):985-96.
41. NICE TA900. Tixagevimab plus cilgavimab for preventing COVID-19; 2023. https://www.nice.org.uk/guidance/ta900/resources/tixagevimab-plus-cilgavimab-for-preventing-covid19-pdf-82615419952837.
42. NHS England. Primary care services. https://www.england.nhs.uk/get-involved/get-involved/how/primarycare/.
43. NHS England. Caring for people at highest clinical risk from COVID-19: Background and FAQs for patients. https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/20200401-FAQs-Patients.pdf.
44. Park S, Elliott J, Berlin A, et al. Strengthening the UK primary care response to covid-19. BMJ. 2020;370:m3691.
45. NICE TA394. Evolocumab for treating primary hypercholesterolaemia and mixed dyslipidaemia; 2016. https://www.nice.org.uk/guidance/ta204/chapter/1-guidance.
46. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500-9.
47. Roth EM, Bujas-Bobanovic M, Louie MJ, Cariou B. Patient and Physician Perspectives on Mode of Administration of the PCSK9 Monoclonal Antibody Alirocumab, an Injectable Medication to Lower LDL-C Levels. Clin Ther. 2015;37(9):1945-54.e6.
48. Pivot X, Gligorov J, Müller V, et al. Patients' preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2-positive early breast cancer: final analysis of 488 patients in the international, randomized, two-cohort PrefHer study. Ann Oncol. 2014;25(10):1979-87.
49. NICE TA659. Galcanezumab for preventing migraine; 2020. https://www.nice.org.uk/guidance/ta659.
50. The Migraine Trust. Calcitonin Gene-Related Peptide (CGRP) monoclonal antibodies. https://migrainetrust.org/live-with-migraine/healthcare/treatments/calcitonin-gene-related-peptide-monoclonal-antibodies/.
51. Tepper SJ, Dahlöf CG, Dowson A, et al. Prevalence and diagnosis of migraine in patients consulting their physician with a complaint of headache: data from the Landmark Study. Headache. 2004;44(9):856-64.
52. Finley CR, Chan DS, Garrison S, et al. What are the most common conditions in primary care? Systematic review. Can Fam Physician. 2018;64(11):832-40.
53. Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the Preventive Treatment of Chronic Migraine. N Engl J Med. 2017;377(22):2113-22.
54. Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm. 2014;20(1):22-33.
55. Lipton RB, Hutchinson S, Ailani J, et al. Discontinuation of Acute Prescription Medication for Migraine: Results From the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache. 2019;59(10):1762-72.
56. Holmes S, Carroll W, Mosgrove F, et al. Primary Care Respiratory Update. Severe asthma – A pragmatic guide for primary care practitioners; 2022. https://www.pcrs-uk.org/resource/severe-asthma-pragmatic-guide-primary-care-practitioners.
57. NICE TA751. Dupilumab for treating severe asthma with type 2 inflammation; 2021. https://www.nice.org.uk/guidance/ta751
58. NICE TA278. Omalizumab for treating severe persistent allergic asthma; 2013. https://www.nice.org.uk/guidance/TA278.
59. Lytsy P, Engström S, Ekstedt M, et al. Outcomes associated with higher relational continuity in the treatment of persons with asthma or chronic obstructive pulmonary disease: A systematic review. EClinicalMedicine. 2022;49:101492.
60. NICE CG153. Psoriasis: assessment and management; 2012 (updated September 2017). https://www.nice.org.uk/guidance/cg153.
61. NHS. Psoriasis: Treatment. https://www.nhs.uk/conditions/psoriasis/treatment/
62. van de Kerkhof PC, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015;29(10):2002-10.
63. Armstrong A, Jarvis S, Boehncke WH, et al. Patient perceptions of clear/almost clear skin in moderate-to-severe plaque psoriasis: results of the Clear About Psoriasis worldwide survey. J Eur Acad Dermatol Venereol. 2018;32(12):2200-7.
64. Langley RG, Lebwohl M, Krueger GG, et al. Long-term efficacy and safety of ustekinumab, with and without dosing adjustment, in patients with moderate-to-severe psoriasis: results from the PHOENIX 2 study through 5 years of follow-up. Br J Dermatol. 2015;172(5):1371-83.
65. Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58(1):106-15.
66. Florek AG, Wang CJ, Armstrong AW. Treatment preferences and treatment satisfaction among psoriasis patients: a systematic review. Arch Dermatol Res. 2018;310(4):271-319.
67. Nelson PA, Barker Z, Griffiths CE, et al. 'On the surface': a qualitative study of GPs' and patients' perspectives on psoriasis. BMC Fam Pract. 2013;14:158.
68. Schofield JK, Fleming D, Grindlay D, Williams H. Skin conditions are the commonest new reason people present to general practitioners in England and Wales. Br J Dermatol. 2011;165(5):1044-50.
69. Fleming P, Yang YB, Lynde C, et al. Diagnosis and Management of Atopic Dermatitis for Primary Care Providers. J Am Board Fam Med. 2020;33(4):626-35.
70. European Medicines Agency. Dupixent; 2017. https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent.
71. European Medicines Agency. Adtralza; 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/adtralza.
72. LeBovidge JS, Elverson W, Timmons KG, et al. Multidisciplinary interventions in the management of atopic dermatitis. J Allergy Clin Immunol. 2016;138(2):325-34.
73. Hudson RDA, Ameen M, George SMC, et al. A Real-World Data Study on the Healthcare Resource Use for Uncontrolled Moderate-to-Severe Atopic Dermatitis in Secondary Care in the United Kingdom Prior to the Introduction of Biologic Treatment. Clinicoecon Outcomes Res. 2022;14:167-77.
74. NICE TA204. Denosumab for the prevention of osteoporotic fractures in postmenopausal women; 2010. https://www.nice.org.uk/guidance/ta204/chapter/1-guidance.
75. Brobst B, Borger J. Benefits And Risks Of Administering Monoclonal Antibody Therapy For Coronavirus (COVID-19). StatPearls. Treasure Island (FL)2022.
76. Hansel TT, Kropshofer H, Singer T, et al. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9(4):325-38.
77. Pichler WJ. Adverse side-effects to biological agents. Allergy. 2006;61(8):912-20.
78. European Medicines Agency. Beyfortus: European public assessment report; 2022. https://www.ema.europa.eu/en/documents/assessment-report/beyfortus-epar-public-assessment-report_en.pdf.
79. Dahal R, Bista S. Strategies To Reduce Polypharmacy in the Elderly. StatPearls. Treasure Island (FL)2023.
80. Aubin F, Carbonnel F, Wendling D. The complexity of adverse side-effects to biological agents. J Crohns Colitis. 2013;7(4):257-62.
81. Kontermann RE. Dual targeting strategies with bispecific antibodies. MAbs. 2012;4(2):182-97.
82. NICE BNF Blinatumomab [Specialist drug]. https://bnf.nice.org.uk/drugs/blinatumomab-specialist-drug/#indications-and-dose.
83. Singh D, Fuhr R, Bird NP, et al. A Phase 1 study of the long-acting anti-IL-5 monoclonal antibody GSK3511294 in patients with asthma. Br J Clin Pharmacol. 2022;88(2):702-12.
84. Levin MJ, Ustianowski A, De Wit S, et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of Covid-19. N Engl J Med. 2022;386(23):2188-200.
85. Caskey M, Kuritzkes DR. Monoclonal Antibodies as Long-Acting Products: What Are We Learning From Human Immunodeficiency Virus (HIV) and Coronavirus Disease 2019 (COVID-19)? Clin Infect Dis. 2022;75(Suppl 4):S530-s40.
86. Centers for Disease Control and Prevention. Antimicrobial Resistance. https://www.cdc.gov/drugresistance/index.html
87. World Health Organization. Antimicrobial resistance;2021. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
88. François B, Jafri HS, Chastre J, et al. Efficacy and safety of suvratoxumab for prevention of Staphylococcus aureus ventilator-associated pneumonia (SAATELLITE): a multicentre, randomised, double-blind, placebo-controlled, parallel-group, phase 2 pilot trial. Lancet Infect Dis. 2021;21(9):1313-23.
89. European Medicines Agency. Zinplava; 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/zinplava.
90. Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55 Suppl 2(Suppl 2):S154-61.
91. Lorenz U, Lorenz B, Schmitter T, et al. Functional antibodies targeting IsaA of Staphylococcus aureus augment host immune response and open new perspectives for antibacterial therapy. Antimicrob Agents Chemother. 2011;55(1):165-73.
92. Vaisman-Mentesh A, Gutierrez-Gonzalez M, DeKosky BJ, Wine Y. The Molecular Mechanisms That Underlie the Immune Biology of Anti-drug Antibody Formation Following Treatment With Monoclonal Antibodies. Front Immunol. 2020;11:1951.
93. NICE TA180. Ustekinumab for the treatment of adults with moderate to severe psoriasis; 2009 (updated 2017). https://www.nice.org.uk/guidance/ta180
94. Gelhorn HL, Balantac Z, Ambrose CS, et al. Patient and physician preferences for attributes of biologic medications for severe asthma. Patient Prefer Adherence. 2019;13:1253-68.
Related articles
View all Articles