Pharmacology for nurses in general practice
Kerri Douglas
Kerri Douglas
RN Dip BSc (Hons) MSc
FHEA Lecturer in Adult Nursing,
Non-Medical Prescribing Lead,
Keele University
Andrew Finney
RN Dip, BSc (Hons) PhD
FHEA Senior Lecturer in Nursing,
Keele University
Practice Nurse 2022;52(5):24-29
All nurses, including those who are not non-medical prescribers, need an understanding of pharmacology if they are to safely carry out their key role in advising about and administering medication
Nurses play a key role in administering medications, even as non-prescribers,1 and a considerable number of general practice nurses are now non-medical prescribers (NMPs). However, it has been suggested that many feel that they lack pharmacological knowledge.2 This is particularly concerning as lack of pharmacology knowledge can lead to medication errors.3
This article provides a guide to a better understanding of pharmacology for general practices nurses (GPNs) and advanced nurse practitioners (ANPs) in primary care.
WHY YOU NEED TO KNOW ABOUT PHARMACOLOGY
GPNs work autonomously and, while they may not all prescribe, they may advise patients about the drugs they are taking. When prescribing or administering medications, whether or not they are NMPs, nurses have a professional responsibility to understand the pharmacology related to those medications in order to practise effectively and, most importantly, to preserve patient safety.4
Nurses are expected to work within professional standards. ‘The Code’ stipulates that nurses must work within their scope of practice. This includes advising on or administering medication within their competence.4
In recent years there have been significant advances in Non-Medical Prescribing (NMP), with nurses engaging in further education to achieve an NMP qualification. Demonstration of an adequate level of pharmacology knowledge is a requirement of any NMP programme,5 but this must not be viewed as something to be developed to ‘get through’ the programme. Rather, it should be regarded as a level of knowledge that must be maintained in order to practise safely.
Nurses who are NMPs remain accountable to their professional body,4 with an additional level of professional responsibility. In 2018 the Nursing and Midwifery Council (NMC) adopted the Royal Pharmaceutical Society (RPS) Competency Framework for all prescribers and voted to continue to use it in 2021 after it was updated.
The RPS Competency Framework is a generic framework that all prescribers should use as guidance for safe and effective prescribing. The framework highlights key competencies that prescribers should have to support safe prescribing, including applying knowledge of pharmacology to their prescribing role.6 For NMPs a lack of pharmacology knowledge increases the risk of inappropriate and unsafe prescribing, with severe, and potentially fatal consequences for patients.7
When advising on or prescribing medication it is important to recognise that the definition of a drug includes medicinal drugs, such as those used for prevention and treatment, non-medicinal drugs, such as those used for recreational purposes, and herbal therapies.8 Drugs of all types can produce adverse effects and interact with each other, leading to unwanted effects.9 For NMPs it is essential to take a thorough drug history from the patient to identify all the drugs the patient is taking. A full assessment aids safe prescribing and reduces the risk of interactions and adverse effects.6 Box 1 details key questions that patients should be asked in order to gain a detailed drug history.
PHARMACOLOGY
Pharmacology is, ‘the study of the actions, mechanisms, uses and adverse effects of drugs’.8 It has two subdivisions, pharmacokinetics and pharmacodynamics.
PHARMACOKINETICS
Pharmacokinetics is ‘what the body does to the drug’. It focuses on how drugs move through the body and has four key elements: Absorption, Distribution, Metabolism and Excretion (ADME).
Absorption
Absorption covers how a drug gets into the circulation so that it can have an effect.
The body provides obstacles to drugs being absorbed which can impact on the amount of drug reaching the circulation intact in order to exert its effects. This is known as the drug’s bioavailability.10
The dose of drug given should be enough to produce a desired effect while avoiding harmful effects, known as toxicity. This is termed ‘keeping the drug within the therapeutic range’ (TR). Many drugs have a large TR, meaning that, if the regime is accurate, the risk of drugs reaching the toxic range is low. Other drugs, such as theophylline, have a narrow TR. Blood levels of such drugs will need to be monitored; if levels fall below the TR the effects of the drug can be reduced; if levels are above the TR there is an increased risk of toxicity.9
It is important to consider the route of administration and the impact this could have on bioavailability. For example, if a drug is given intravenously administration is directly into the circulation. There would be no barriers to the process of absorption and the drug would have 100% bioavailability. If drugs are administered via another route, the process of absorption will reduce the bioavailability.11
When taken orally some drug will be absorbed through the stomach lining. For many drugs, however, absorption will occur in the small intestine.12 There are factors that can impact the effectiveness of absorption of oral drugs:
- Gastric content – the presence of food can reduce the absorption of some drugs. Some antibiotics need to be taken on an empty stomach due to the impact food in the gastrointestinal tract can have on absorption.11
- Gastric pH – contact with gastric acid can render some drugs inactive and unable to have any effect. Insulin is an example.13
- Concurrent drug administration – some drugs, when administered concurrently, can bind together, creating a large molecule that can be difficult to absorb. As a result neither drug is absorbed in the amounts expected. Examples of two such drugs are doxycycline and calcium.
Once orally administered drugs have been absorbed they are taken directly to the liver via the hepatic portal vein, where they go through the process of first pass metabolism. First pass metabolism, primarily but not exclusively associated with the liver, is the process that begins to break down drugs before they enter the circulation. A key consideration is that many drugs start being broken down during first pass metabolism, leaving a reduced amount of drug available to have an effect.14 There are specific drugs that cannot be administered orally as the process of first pass metabolism is so extensive that bioavailability is significantly reduced. Glyceryl trinitrate is an example.15
Distribution
Distribution is the process of drugs dispersing and passing through body tissues and fluids.12
Once a drug has been through the process of absorption it must be distributed to its site of action. Drugs administered via inhalation are delivered directly to the site of action, the lungs.16 For orally administered drugs, the process of distribution is required for the proportion of drug that remains active following first pass metabolism to be taken to its site of action.
When drugs are within the blood stream, part of the drug will bind to plasma protein, known as bound drug. Some will remain free within the plasma, known as free drug. The drug that is bound is unable to leave the blood stream to exert its effects. The free drug can leave the plasma and move to the body cells to exert its effect.12 As the free drug begins to move to body tissues the bound drug can release and become free.
One of the factors that could affect the process of distribution, by impacting the balance between bound and free drug, is a low plasma protein level. There is less protein for drugs to bind too, resulting in a higher-than-expected amount of drug that is free. Age-related factors or fluid levels within the body can effect plasma protein levels. Patients with underlying disease may have hypoalbuminemia which can lead to greater amounts of free drug.9
If a patient is on more than one drug, the drugs can compete to bind to plasma proteins. Some drugs have a stronger attraction, known as affinity, to the plasma proteins than others, resulting in the other drug being displaced. This can produce an increase in the amount of the drug with less affinity being free. An example of a drug with multiple interactions is warfarin. There are many drugs that can displace warfarin from plasma proteins, resulting in a greater amount of free warfarin. This produces a greater anticoagulant effect and a risk of bleeding.
The body has a protective element to prevent many drugs passing from body fluids to the brain, known as the blood-brain barrier. Many drugs are not able to cross this barrier, but some can, potentially leading to neurological side effects. For example, chlorphenamine can cause drowsiness.12 If the sedative effects of antihistamines are likely to be problematic a non-sedating anti-histamine should be selected instead, as they are not able to cross the blood-brain barrier.
Metabolism
Metabolism of drugs occurs predominantly in the liver. As drugs move through the circulation they reach the liver, which attempts to break them down to make them soluble and therefore easier to be excreted by the body. It is important to note that the proportion of the drug that is free can be metabolised; drug that is bound to plasma protein will not be.
Metabolism occurs in two phases, phase one and phase two. Phase one metabolism involves enzymes within the liver. These work to break down the chemical substance of the drug. In phase two the metabolites produced in phase one are conjugated to make them more water soluble and easier to excrete.
Drug interactions can occur during metabolism and drugs can compete for the enzymes involved. If this competition occurs it is possible that the more competitive drugs will be broken down by the enzymes and the less competitive of the drugs may not be metabolised as expected. The less competitive drug, therefore, remains in the circulation for longer than expected. Clarithromycin and simvastatin are examples of drugs that can interact during metabolism.15
Patients with hepatic impairment may metabolise drugs at different rates than expected. This can impact on how much of the drug is metabolised in comparison with what is expected. Therefore, the doses of hepatotoxic drug may need to be adjusted.9
While metabolism aims to break down drugs, for some drugs the process of metabolism ‘activates’ them. These are known as pro-drugs. A common example of a pro-drug is codeine which, when metabolised, converts to morphine which is then able to exert its effects.11
Excretion
The kidneys are the predominant site for drug excretion. However, excretion can occur in body secretions such as breast milk and this would need to be considered in the event of prescribing to breastfeeding mothers.
As plasma reaches the glomerulus, water, electrolytes and drug metabolites begin to be filtered out. It is again important to recognise that this process will only happen with the free drug, therefore there can be several passes of drugs through the kidneys before they are fully excreted.
In renal impairment, excretion of drugs may be reduced. This has the potential to increase side effects, and even toxicity. Delayed excretion can cause drugs to accumulate, and half-life can increase.9 In contrast, some drugs may become ineffective in those with renal impairment. These are drugs which are ‘activated’ by the kidneys into a substance that can take effect. If renal function is reduced activation may be inadequate. An example of this is vitamin D3.15
Drugs which rely on accumulation in urine to exert their effect can become inactive in renal impairment. For example, nitrofurantoin is commonly used to treat urinary tract infections. To be effective it requires a level of concentration in the urine.17 If renal function is impaired the drug may not attain adequate levels and its effectiveness will therefore be reduced.
It is also important to remember that there is a natural decline in renal function associated with aging. Elderly patients man have reduced renal function and they are at greater risk of acute kidney injury.18
Box 2 summarises the patient groups that may require special consideration when prescribing.
PHARMACODYNAMICS
Pharmacodynamics is ‘what the drug does to the body’. Drugs produce their effect by interacting with targets, binding to them to elicit a change in their function.19 Most of the binding sites for drugs are proteins and there is a wide range of proteins within the body.
Targets of drug action
There are several targets of drug action. Examples include receptors, enzymes, ion-channels and transport systems.
Receptors on cells are common targets for drug action. As drugs move through the systemic circulation, free drug can interact with receptors on the cell surface. This interaction allows the receptor to convert the drug into something that the cell can recognise and respond to.11
Enzymes within the body maintain the functioning of cells by impacting upon the speed of chemical reactions. Drugs that act on enzymes bind to them and can alter the activity of the enzyme itself. Most drugs that act on enzymes inhibit their activity, reducing its action. An example is the non-steroidal anti-inflammatory drugs (NSAIDS). These act on the cyclo-oxygenase (COX) enzyme. The chemical responses associated with COX enzymes include an inflammatory response and NSAIDS act to inhibit the enzyme’s action.20
Ion-channels are protein complexes that facilitate the flow of ions, such as sodium, potassium, and calcium, across cell membranes. Without the channel, these ions would not be able to pass through. Drugs can impact on the ion-channel in different ways. A common example is a drug which acts to block the channel, preventing the passage of ions into the cell.21 Amlodipine is a calcium channel blocker. It works by blocking the influx of calcium into cardiac and vascular smooth muscle. This results in relaxation of smooth muscle and a reduction of resistance, producing an antihypertensive effect.15
Drugs can exert their action by interacting with transport systems. These are the carriers that can move drugs from one side of a cell membrane to the other. An example of a drug that works by interacting with a transport system is citalopram. This belongs to the drug group Selective Serotonin Reuptake Inhibitors (SSRIs). Following release of the chemical serotonin into the body it is rapidly taken back to nerve endings by transporter proteins. Citalopram inhibits these transporter proteins, preventing the rapid uptake of serotonin so that serotonin is available for longer. The increased presence of serotonin can improve mood and is why SSRIs, such as citalopram, are used to treat depression.21
An important thing to remember is that, while drugs may have specificity to certain receptors, these receptors may be found on multiple cells within the body. For example opioids will bind to opioid receptors both in the nervous system and GI system. The nervous system receptor binding will provide the desired effect of pain relief. However the binding to opioid receptors in the GI system can reduce motility resulting in unwanted side effects such as constipation.22
Some drugs bind more tightly to a receptor than others, i.e. they have a ‘high affinity’ to a receptor. Such high affinity drugs will be detected in low concentrations by the receptor and a lower dose of the drug will therefore be required in order to produce a therapeutic effect. In the case of two drugs being administered together, where one drug has a higher affinity to a receptor than the other, it is likely that the lower affinity drug will be displaced and will not be able to produce the desired therapeutic effect.23
Drug effect at target site
Drugs are typically described as having an agonist or antagonist effect at their target site.
A drug that is considered an agonist binds to its receptor and activates the receptor to produce a response. An example is salbutamol, a beta-2 agonist drug. Beta-2 receptors are found in bronchial smooth muscle. When the receptors are activated by salbutamol the response is relaxation of the smooth muscle and relief of bronchospasm.15 However, beta-2 receptors are also found in several other places, such as skeletal and cardiac smooth muscle. Salbutamol can interact with these receptors too, resulting in patients experiencing unwanted side effects, such as tremor associated with an interaction with skeletal muscle and tachycardia associated with interaction with cardiac smooth muscle.24
Some drugs, known as partial agonists, are agonists but only have a partial effect. They may not be as effective as full agonists but they can still be beneficial drugs. Buprenorphine is an example of a partial opioid agonist. It has some effect on the opioid receptors and is often used in the treatment of opioid dependence.15
Antagonist drugs bind to a receptor and block or reduce its response. Montelukast is an example of an antagonist drug. Leukotrienes are one of several inflammatory molecules released during the inflammatory response seen in asthma. The effect of this inflammatory response is mucous production and bronchoconstriction. Montelukast, a leukotriene receptor antagonist, works by interacting with leukotriene receptors to reduce the inflammatory responce.15
Beta-blockers are a common example of a receptor antagonist. They work by interacting with beta-receptors to reduce the response to adrenaline. Their effect on cardiac beta receptors can reduce the heart rate and relax smooth muscle which can help to lower blood pressure.25 As we have already noted, receptors are not necessarily confined to the target organ and antagonist drugs can produce unwanted side effects. For example, beta 2 receptors are present in bronchial smooth muscle. A potential adverse effect of beta-blockers for patients with respiratory conditions, such as asthma and COPD, is bronchoconstriction. Some beta-blockers are known as cardio-selective and will therefore target the cardiac receptors specifically, but specificity can be relative. They should still only be used in those with respiratory conditions when they are clearly indicated.26
SUMMARY
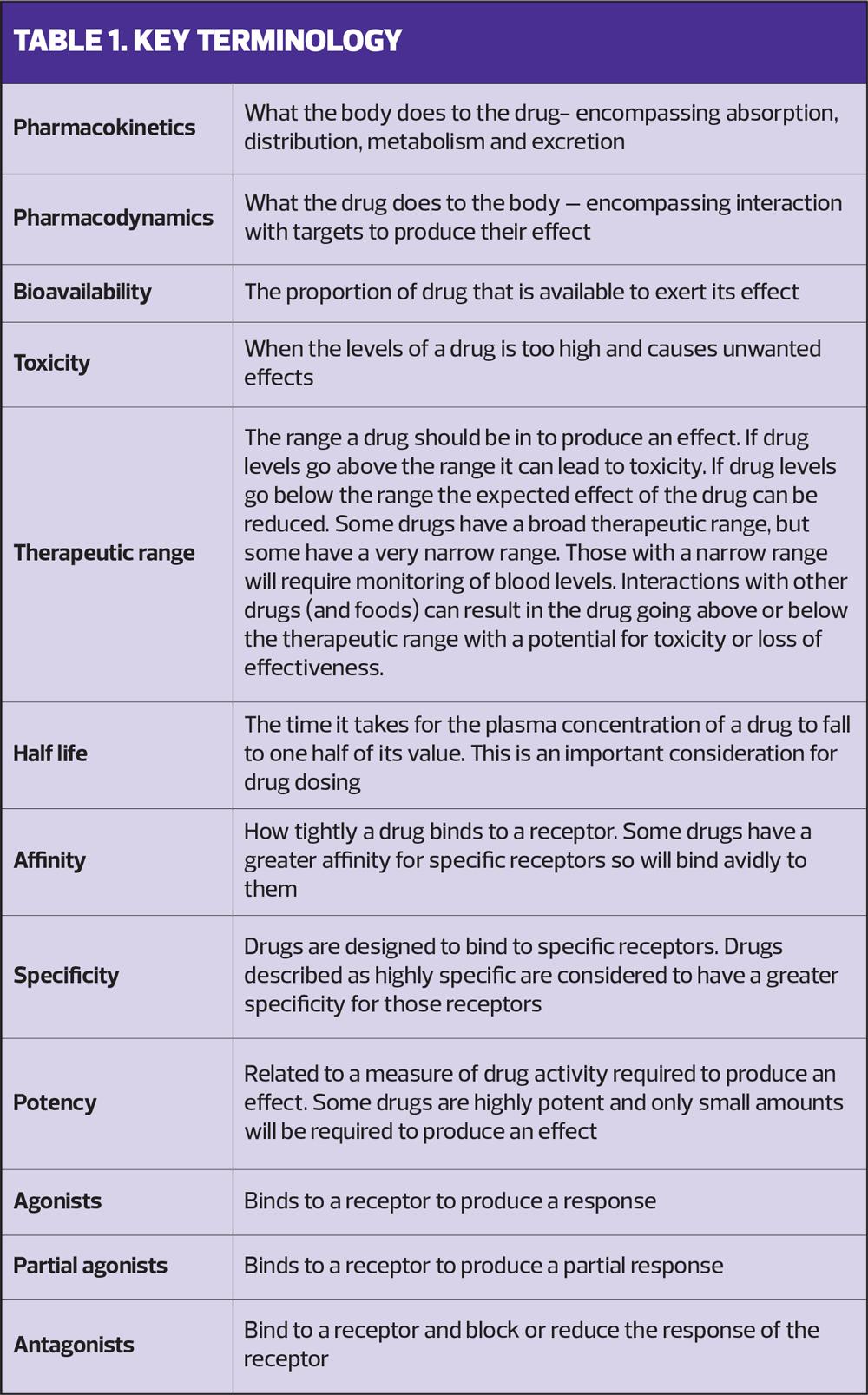
Pharmacology is broken down into pharmacokinetics and pharmacodynamics and Table 1 gives a summary of the terminology used.
The process of how drugs move through the body, and what they do to the body, can be influenced by many factors that require consideration in order to reduce the risk of adverse effects for patients. Nurses require an understanding of pharmacology to ensure that medications are prescribed, administered and advised on safely. In doing so this will maximise the benefits of the medications whilst reducing risks and will ensure compliance with professional standards.
REFERENCES
1. Cunningham M, Tucker S. Pharmacology in healthcare, support for nurses and midwives. British Pharmacological Society; 2020 https://www.bps.ac.uk/publishing/blog/may-2020/pharmacology-in-healthcare-support-for-nurses-an
2. Ndosi ME, Newell R. Nurses' knowledge of pharmacology behind drugs they commonly administer. J Clin Nurs 2009; 18(4): 570–620.
3. Simonsen BO, Daehlin G K, Johansson I, et al. Differences in medication knowledge and risk of errors between graduating nursing students and working registered nurses: comparative study. BMC Health Serv Res 2014; 14: 580. https://doi.org/10.1186/s12913-014-0580-7.
4. Nursing & Midwifery Council. The code: Professional standards of practice and behaviour for nurses, midwives and nursing associates; 2018 https://www.nmc.org.uk/standards/code/
5. Nursing & Midwifery Council. Standards for prescribing programmes; 2019. https://www.nmc.org.uk/standards/standards-for-post-registration/standards-for-prescribers/standards-for-prescribing-programmes/
6. Royal Pharmaceutical Society. A Competency Framework for Designated Prescribing Practitioners; 2021 https://www.rpharms.com/resources/frameworks/prescribers-competency-framework
7. Avery AJ, Coleman JJ. Tackling potentially inappropriate prescribing. BMJ 2018;363:k4688 doi:10.1136/bmj.k4688.
8. Catrin P. Crash Course Pharmacology. 5th edition. Elsevier: London; 2019
9. British National Formulary, Joint Formulary Committee; 2021 https://bnf.nice.org.uk/.
10. Golan DE, Armstrong E J, Tashjian A H, et al. Principles of pharmacology: the pathophysiologic basis of drug therapy. 3rd edition. Lippincott: USA; 2011
11. Page CP, Anand R, DeWilde S, et al. Trounce’s pharmacology for nurses and allied health professionals. 19th edition. Elsevier: London; 2022
12. Ashelford s, Raynsford J, Taylor V. Pathophysiology and pharmacology for nursing students. Sage: London; 2016
13. Lopes M, Derenne A, Pereira C, et al. Impact of the in vitro gastrointestinal passage of biopolymer-based nanoparticles on insulin absorption. RSC Advances 2016: 6(24): 20155-65.
14. Willihnganz MJ, Clayton BD, Gurevitz SL. Clayton’s basic pharmacology for nurses. 18th edition. Elsevier: USA; 2019
15. Electronic Medicines Compendium (EMC); 2022. https://www.medicines.org.uk/emc/
16. Barber P, Parkes J, Blundell D. Further essentials of pharmacology for nurses. McGraw-Hill Education/Open University Press; 2012
17. Squadrito FJ, del Portal D. Nitrofurantoin. [Updated 2021 Jul 13]. In: StatPearls https://www.ncbi.nlm.nih.gov/books/NBK470526/.
18. Fusco S, Garasto S, Corsonello A, et al. Medication-Induced Nephrotoxicity in Older Patients. Curr Drug Metab 2016;17(6):608–625.
19. Marino M, Jamal Z, Zito PM. Pharmacodynamics. [Updated 2022 Jan 11]. In: StatPearls https://www.ncbi.nlm.nih.gov/books/NBK507791/.
20. Fitzpatrick FA. Cyclooxygenase enzymes: regulation and function. Curr Pharm Des 2004; 10(6): 577–588. https://doi.org/10.2174/1381612043453144.
21. Robertson D. Introduction to pharmacodynamics. Nurse Prescribing 2017;15(4):198-200 https://doi.org/10.12968/npre.2017.15.4.198
22. Roy K. In Silico drug design. Repurposing techniques and methodologies 1st edition. Elsevier: USA; 2019
23. Lambert DG. Drugs and receptors. Continuing Education in Anaesthesia Critical Care & Pain 2004;4(6):181-4 https://doi.org/10.1093/bjaceaccp/mkh049
24. Fowler SJ, Lipworth BJ. Pharmacokinetics and systemic beta2-adrenoceptor-mediated responses to inhaled salbutamol. BJ Clin Pharm 2001;51(4):359-62 https://doi.org/10.1046/j.1365-2125.2001.01362.x.
25. Klabunde RE. Cardiovascular Physiology Concepts. 3rd edition. Wolters Kluwer: China; 2022
26. Morales DR, Lipworth BJ, Donnan PT, et al. Respiratory effect of beta-blockers in people with asthma and cardiovascular disease: population-based nested case control study. BMC Med 2017;15(1):18. https://doi.org/10.1186/s12916-017-0781-0
Related articles
View all Articles