Antimicrobial resistance: has anything changed?
Ross Ferguson
Ross Ferguson
BSc MFRPSI MRPharmS
Assistant Editor, Clarity Informatics
Practice Nurse 2020;50(7):28-33
Less than two decades after the discovery of penicillin in 1928, Sir Alexander Fleming warned that misuse of the drug could result in selection for resistant bacteria. Fast forward to 2013 and the Chief Medical Officer for England, Dame Sally Davies, launched an antimicrobial resistance strategy to combat this ‘catastrophic threat’. But was it too little too late?
Antimicrobial resistance (AMR) is caused by bacteria losing sensitivity to antibiotics, but it is important to note that this also occurs in relation to resistance to treatment of viruses, fungi, worms, and protozoa. Bacterial resistance occurs naturally but is accelerated with use and overuse of antibiotics.
AMR can occur in relation to life-threatening bacterial infections, including pneumonia and meningitis. This can result in these infections being resistant to treatment using existing antibiotics (for example, methicillin-resistant Staphylococcus aureus), or result in previously minor infections becoming major threats to health and wellbeing. AMR is a global health problem, and it is estimated that it causes around 700,000 deaths worldwide per year — worryingly this is predicted to rise to 10 million by 2050 if action is not taken.1
FOLLOWING THE SCRIPT
In England in 2012, 43.3 million prescriptions were issued for antibiotics. This number fell to 37.1 million in 2017, a fall of (14.4%).2 Yet, antibiotic-resistant bloodstream infections increased from 12,972 in 2014 to 17,108 in 2018, and antibiotic-resistant infections in general rose from 55,812 in 2017 to 61,000 in 2019 — a rise of 9%.3
The vast majority of antibiotic prescriptions (80%) are issued in primary care and a study has revealed that sore throat, cough, sinusitis, ear infection and urinary tract infections were the conditions most associated with potentially inappropriate prescribing of antibiotics.4
It is estimated that at least 20% of all antibiotic prescriptions in primary care may be inappropriate. This contributes to antimicrobial resistance, which could mean that in the future, simple infections may not be treatable with antibiotics, resulting in increased morbidity and mortality.4
In fact, the scale of antibiotic prescribing in primary care has been shown to directly and adversely affect antimicrobial resistance — a 2010 systematic review and meta-analysis of studies which investigated subsequent antibiotic resistance in people prescribed antibiotics in primary care found that individuals prescribed an antibiotic in primary care for a respiratory or urinary infection develop bacterial resistance to that antibiotic.5
The effect is greatest in the month immediately after treatment but may persist for up to 12 months, and it not only increases the population carriage of organisms resistant to first-line antibiotics but also creates the conditions for increased use of second-line antibiotics in the community. Longer duration and multiple courses of antibiotics were associated with higher rates of resistance.5
Put simplistically, there are two ways to tackle AMR — develop new antibiotics, or make better use of the antibiotic that are currently available.
END OF THE LINE?
Incredibly, against a backdrop of significant medical advances in the last half-century, no new classes of antibiotic have been discovered since the 1980s. There are currently 60 products in development (50 antibiotics and 10 biologics), but these drugs are likely to bring only small benefits over existing treatments and few target the most critical infections (for example, gram-negative bacteria).6
And, while there are over 250 antimicrobial agents in early-stage testing it will take up to 10 years for the first to become commercially available. At the moment, therefore, we cannot rely entirely on new treatments.
As you can imagine, the drug discovery and development process is far from inexpensive — it costs over $1 billion to develop a drug and bring it to market. Pharmaceutical companies claim that the incentives are no longer there, with some manufacturers saying that the economic model is failing.7
Concern about the lack of innovation in the development of new antimicrobial treatments and declining investment led the World Health Organization (WHO) to develop two documents earlier this year: Target product profiles for needed antibacterial agents, to guide development of new treatments for common resistant bacterial infections and A financial model for an impact investment fund for the development of antibacterial treatments and diagnostics, an economic model that simulates the costs, risks, and possible return on investment of antibacterial drug development.
Meanwhile in June this year, the UK Government launched a new scheme that aims to incentivise companies to invest in researching and developing new antibiotics.8 The payment model works differently from the current one – it offers to pay pharmaceutical companies in advance to develop new antibiotics, and rather than pay companies for the quantity of product the NHS uses, the scheme will pay for access to the antibiotic based on its value to the NHS.
Two drugs that have proven to be both safe and effective will be selected at the end of 2020 to undergo health technology assessment (HTA) by the National Institute for Health and Care Excellence (NICE) throughout 2021 using adapted methods for antimicrobials. The HTA will then be used to decide the level of the subscription payment.
PEAK PRACTICE
Given the parlous state of drug development, strategies for improving infection prevention and control, and ensuring existing antibiotics are used appropriately are key to securing future treatment efficacy. This brings into sharp focus the importance of clinical guidelines and in particular antibacterial prescribing guidelines.
In 2013, of the 79 million prescriptions dispensed in England, approximately 18 million items (23%) were prescribed by nurses.9 It is clear that practice nurses have a significant role in ensuring that antibiotics are used appropriately in line with clinical guidelines and antimicrobial prescribing policies.
The NICE key therapeutic topic Antimicrobial stewardship: prescribing antibiotics is an essential read for any nurse prescriber, as is the Public Health England (PHE) Antimicrobial prescribing and stewardship competencies document.
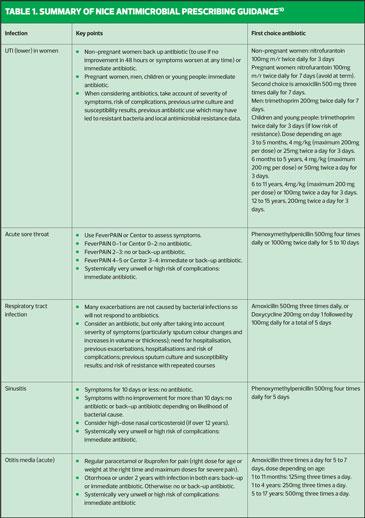
NICE antimicrobial prescribing guidelines exist for many conditions, including those most associated with inappropriate prescribing of antibiotics. It is important for practice nurses to be aware of these guidelines as they outline circumstances in which antibiotics are unnecessary, when they should be prescribed, preferred options, as well as the dose and length of treatment.
- Sore throat (acute): antimicrobial prescribing https://www.nice.org.uk/guidance/ng84
- Respiratory tract infections (self-limiting): prescribing antibiotics https://www.nice.org.uk/guidance/cg69
- Sinusitis (acute): antimicrobial prescribing https://www.nice.org.uk/guidance/ng79
- Otitis media (acute): antimicrobial prescribing https://www.nice.org.uk/guidance/ng91
- Urinary tract infection (lower): antimicrobial prescribing https://www.nice.org.uk/guidance/ng109
Table 1 summarises the management options for these five common conditions. A more comprehensive table summarising the antimicrobial prescribing guidance for managing common infections is available on the NICE website.10
As you can see, there is no shortage of insight into the causes and contributory factors to AMR as well as guidance and support to combat it. Despite this, barriers still exist in tackling AMR.
BEHAVIOURAL BARRIERS
While nobody could disagree with the practice of antimicrobial stewardship, there are a number of behavioural barriers that need to be considered and overcome. These can be broadly categorised as prescriber, patient or societal factors (Box 1).9
Focussing on the role of prescribers, there can be many contributory factors which lead to inappropriate prescribing, including:9,11
- A decision to prescribe based on ‘gut feeling’ rather than clinical presentation.
- Concerns about having done ‘nothing’ for the patient.
- The desire to maintain a good relationship with the patient.
- Concerns that the patient will not be satisfied and may return to the practice.
- Missing a diagnosis and the possible medicolegal implications.
- A previous bad experience of not prescribing antibiotics.
- Difficulty in differentiating between viral and bacterial infections.
- Work pressure and fatigue.
- Timing of consultations (for example, Friday evening, bank holiday weekend consultations) — one study reported a 23% increase in antibiotic prescribing on Fridays, attributed to uncertainty about treatment accessibility over the weekend.
An awareness of these barriers (and others that may apply to your practice), and applying techniques and processes for avoiding or managing them are part of the challenge of tackling AMR.
COVID CONCERNS
As steady as progress appears to be in the fight against AMR a new threat has emerged from left field. There can be few areas of life that have not been negatively impacted by the COVID-19 pandemic, and there is evidence that antimicrobial resistance is one of the few beneficiaries as normal health service are disrupted and clinicians care for people with the infection.
In fact, the World Health Organization (WHO) has highlighted several potential threats to combating AMR during the pandemic:12
- People presenting with mild or moderate disease and no pneumonia receive antibiotics – a review of studies published on hospitalised COVID-19 patients identified that while 72% (1450/2010) of patients received antibiotics, only 8% (62/806) demonstrated superimposed bacterial or fungal co-infections.
- Hospital admissions increase the risk of healthcare-associated infections and transmission of multidrug-resistant organisms — a recent study conducted in intensive care units in 88 countries showed that although only 54% (8135/15 165) of patients had suspected or proven bacterial infection, 70% (10,640/15,165) of them had received at least one antibiotic either for prophylaxis or treatment purposes.
- Disruption to normal services and treatments (e.g. tuberculosis and HIV) can lead to selection for drug resistance.
- Disruption to vaccinations can increase the risk of infection and potential overuse of antimicrobials.
- Widespread use of biocidal agents for environmental and personal disinfection.
The WHO’s guidance on management of COVID-19 now includes antibiotic stewardship principles with specific recommendations.13 The organization recommends that only people with suspected or confirmed severe COVID-19 infection are given antibiotics, based on clinical judgment, and that that empiric antibiotic bacterial pneumonia treatment can be considered in older people residing in long-term care facilities and children younger than five years with moderate COVID-19.
The WHO argues that antibiotic stewardship should be an integral part of the global response to the pandemic and that five measures are required:
- Increase clinical competence among health workers treating COVID-19 patients.
- Ensure the continuity of essential health services and regular supply of antimicrobials including antiretroviral and tuberculosis drugs and vaccines.
- Reduce the turnaround time of COVID-19 testing.
- Exercise maximum caution in the use of biocides for environmental and personal disinfection.
- Address gaps in research to ensure that antimicrobial stewardship activities become an integral part of the pandemic response and beyond.
CONCERTED EFFORT
The threat of AMR should not be underestimated. If anything, the events of this year caused by a novel coronavirus may have been the added impetus we all needed to refocus our determination to fight AMR.
While there are many strands to the global fight, at practice level individuals need to do their bit to ensure that we make the best use of the available antibiotics while we hope that initiatives to develop new ones are successful, enabling us to continue to fight existing threats and prepare for any new ones. Perhaps the first step is to reflect on your own practice.
REFERENCES
1. HM Government. Contained and controlled: the UK’s 20-year vision for antimicrobial resistance; 2019 https://www.gov.uk/government/publications/uk-20-year-vision-for-antimicrobial-resistance
2. Walker AJ, Curtis HJ, Goldacre B. Impact of Chief Medical Officer activity on prescribing of antibiotics in England: an interrupted time series analysis. J Antimicrob Chemother 2019;74:1133-1136.
3. Public Health England. English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) report; 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/843129/English_Surveillance_Programme_for_Antimicrobial_Utilisation_and_Resistance_2019.pdf
4. NICE. NICE Impact antimicrobial resistance; 2018. https://www.nice.org.uk/media/default/about/what-we-do/into-practice/measuring-uptake/niceimpact-antimicrobial-resistance.pdf
5. Costello C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010;340:c2096
6. WHO. Lack of new antibiotics threatens global efforts to contain drug-resistant infections; 2020. https://www.who.int/news-room/detail/17-01-2020-17-01-2020-lack-of-new-antibiotics-threatens-global-efforts-to-contain-drug-resistant-infections
7. Wellcome. Why is it so hard to develop new antibiotics? 2020. https://wellcome.ac.uk/news/why-is-it-so-hard-develop-new-antibiotics
8. HM Government. World-first scheme underway to tackle AMR and protect UK patients; 2020. https://www.gov.uk/government/news/world-first-scheme-underway-to-tackle-amr-and-protect-uk-patients
9. PHE. Behaviour change and antibiotic prescribing in healthcare settings: literature review and behavioural analysis; 2015. https://www.gov.uk/government/publications/antibiotic-prescribing-and-behaviour-change-in-healthcare-settings
10. NICE. Summary of antimicrobial prescribing guidance – managing common infections;2020
11. Germeni E, Frost J, Garside R, et al. Antibiotic prescribing for acute respiratory tract infections in primary care: an updated and expanded meta-ethnography. Br J Gen Pract 2018;68(674):e633-e645.
12. WHO. Tackling antimicrobial resistance in the COVID-19 pandemic; 2020. https://www.who.int/bulletin/volumes/98/7/20-268573/en/
13. WHO. Clinical management of COVID-19; 2020. https://www.who.int/publications/i/item/clinical-management-of-covid-19
Related articles
View all Articles