Improving diagnosis of dementia – the role of the practice nurse
Zena Aldridge
Zena Aldridge
Admiral Nurse Research Fellow, PhD student
De Montfort University, Independent Dementia Nurse Consultant, Norwich.
Dr Karen Harrison Dening
Head of Research & Publications, Honorary Professor of Dementia, De Montfort University
Dementia is an umbrella term used to describe a group of symptoms characterised by behavioural changes and loss of cognitive and social functioning caused by progressive neurological disorders. There are significant advantages to be gained from a timely diagnosis, and practice nurses can play a key role in early identification
Historically, dementia services have been placed in mental health services. This association may have added to the stigma and misconceptions surrounding dementia, its causes and effects. Although the majority of patients are diagnosed within memory assessment services, access to these services requires a referral from primary care and, unless the patient’s needs are considered to be complex in the context of their mental health, the long-term management of the condition is undertaken in primary care.
Dementia diagnosis has been seen by some as a ‘tick box exercise’ but there are significant benefits of a formal diagnosis for patients and their families, including the ability to participate in advanced care planning, improved decision making and management of co -morbid conditions as well as enabling access to specialist support and information about the condition. Practice nurses play a key role in identifying patients who may have the signs and symptoms of dementia by enabling them to access a timely diagnosis.
Dementia is an umbrella term used to describe a group of symptoms characterised by behavioural changes and loss of cognitive and social functioning caused by progressive neurological disorders.1 There are over 200 subtypes of dementia, but the most common are:
- Alzheimer's
- Vascular
- Lewy Body
- Mixed (often a combination of Alzheimer's and vascular), and
- Frontotemporal dementias.
There are estimated to be 850,000 people living with dementia in the UK. If current figures relating to incidence and prevalence are realised, this will increase to 1 million people by 2025 and 2 million by 2051.2 While dementia is largely associated with old age, of those 850,000 people living with dementia there are 42,325 people under 65 years diagnosed with the condition.2 There also estimated to be 700,000 informal, primary carers supporting people with dementia who save the health and social care economy £11.6 billion per year.2 Due to their progressive nature, dementia and Alzheimer’s disease are now the leading causes of death in England and Wales, accounting for 12.0% of all deaths registered in 2016, up from 11.6% in 2015.3
ACCESS TO DIAGNOSIS
In 2015 a key target of the Prime Minister’s ‘Challenge on Dementia 2020’4 was to enable equal access to diagnosis for everyone. Of the 850,000 people estimated to have dementia in the UK, the target is to achieve a diagnostic rate of 66.7%.2 Although this target has been achieved nationally, data from NHS digital demonstrated that diagnosis rates vary hugely across the country, with significant variation across CCGs, ranging from 52.1% in some areas to 89.8% in others in December 2018.5 The NICE guideline on dementia (NG97) acknowledges the complexity of dementia and makes recommendations for high quality assessment, management and ongoing support for people living with dementia, and their families.6 Of course, in order to access management and support, people with dementia need access to a timely diagnosis. Therefore, staff training, enabling understanding and recognition of the varied symptoms of dementia and improved competence and confidence to act upon this knowledge, is fundamental in ensuring that people living with dementia and their families are able to get the support they need and deserve.
SIGNS AND SYMPTOMS OF DEMENTIA
There is an erroneous belief that dementia is solely about memory loss. However, the main diagnostic systems in current use, ICD-107 and the American DSM-V8, give us broader-ranging criteria to determine whether there is a dementia syndrome:
- Multiple cognitive deficits – problems in more than one cognitive domain, such as memory, language, spatial orientation, organisational skills etc. Amnesia or memory impairment for learning new information or recalling previously learned information must be one of the core features.
- Functional impairment – difficulty in maintaining the ability to perform routine activities, at work, or at home or socially, due to the cognitive deficits.
- Change from a previous level – there must be a clear decline in this functional impairment when compared to previous abilities, with progressive decline.
- Clear consciousness – the person must be alert and without any disturbance in consciousness. Altered levels of consciousness can occur in acute confusional states or delirium.
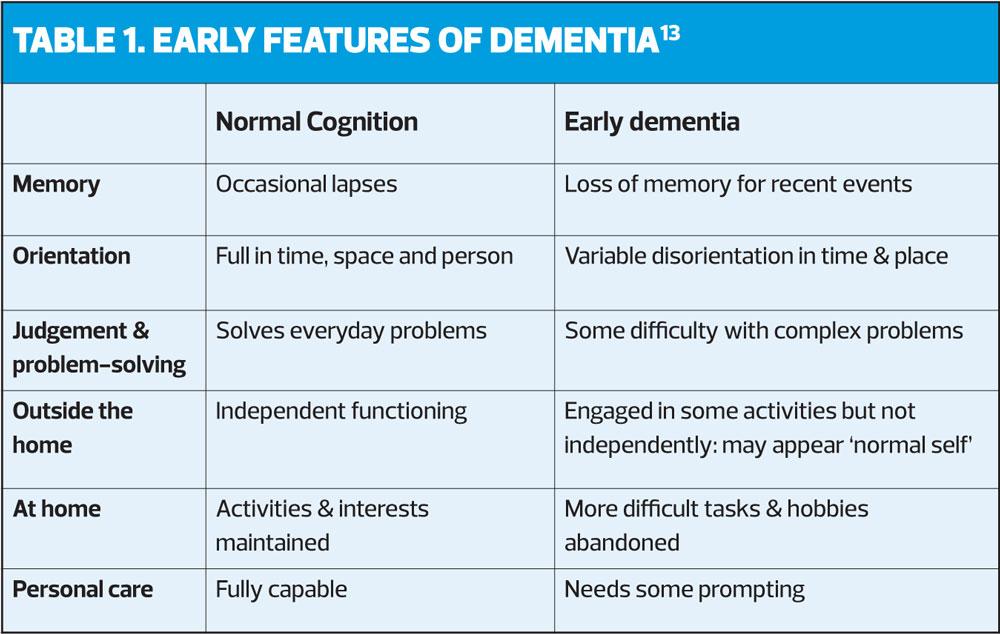
General practice is generally the first point of contact for those who are concerned that they, or a family member may have dementia.9 However, there may be a delay in reporting symptoms due to the stigma attached to the condition or, there may be a lack of awareness of the early symptoms and changes (Table 1).10 Often a patient may not present with concerns about their memory but attend with another complaint or problem.
Therefore, it is important that general practice nurses have a robust knowledge of dementia and its effects, and of some of the differential diagnoses, such as delirium and depression. Recognition of dementia, particularly in the early stages, is not straightforward due to the variation of symptoms. Some symptoms are not obvious and therefore a good understanding of the subtypes of dementia and their respective symptoms can enable early identification, facilitating a timely diagnosis, appropriate support and symptom control. Dementia is the most feared condition in the over-50s11 and it may be that having an improved knowledge of the condition and its early presenting signs can offer reassurance and reduce anxiety in patients who may be concerned that they have dementia. Of equal importance is the opportunity to identify and treat reversible conditions in patients presenting with symptoms that appear indicative of dementia, for example, thyroid dysfunction or nutritional deficiency. Openly discussing dementia symptoms and differential diagnosis may help to reduce stigma and give you the opportunity also to identify if a patient is depressed, emotionally distressed or has another medical, reversible cause of cognitive decline other than dementia.
DEMENTIA, COMORBIDITY AND MULTIMORBIDITY
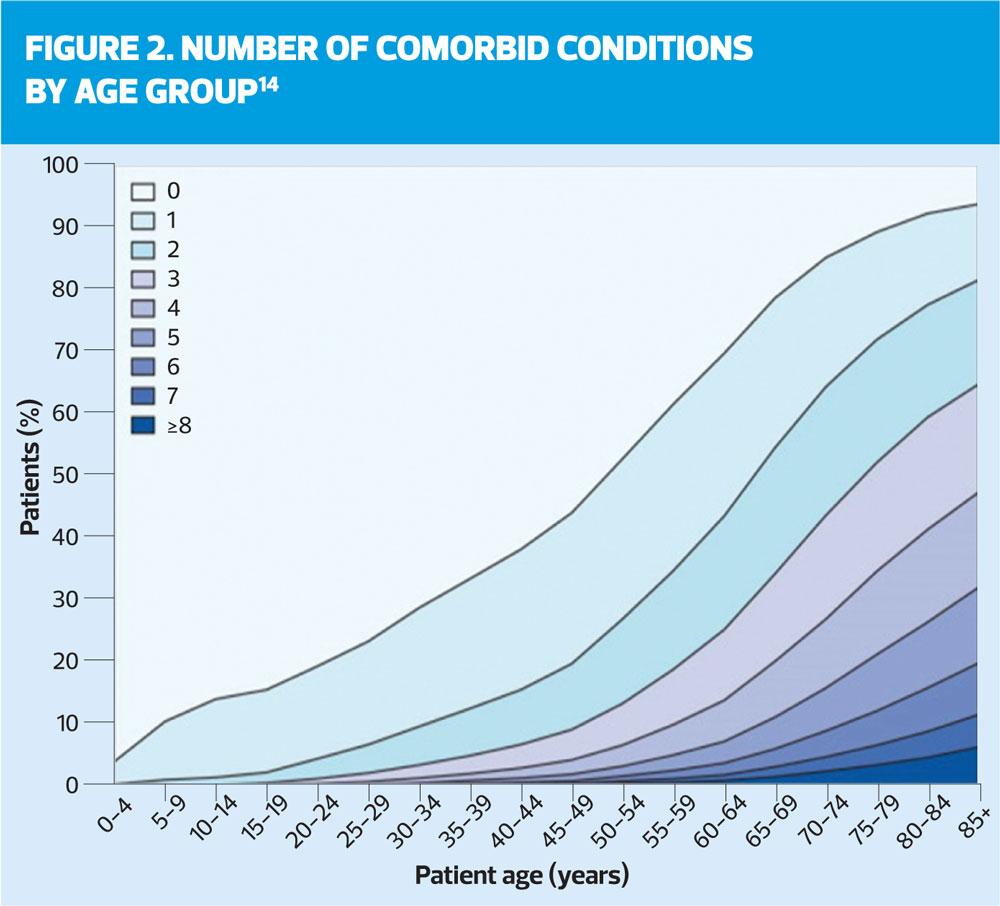
The likelihood of comorbidity and multimorbidity increases with age (Figure 1). Comorbidity can be defined as two or more chronic conditions; multimorbidity is a newer term for multiple conditions that also encompasses medications, lifestyle behaviours, disability, and socioeconomic stressors. A large part of practice nurse work is about monitoring long term conditions in older people, which puts you in a position to identify the emergence of cognitive impairment, even if you or the patient do not at first register the significance of it. The conversations that you have with your older patients, while checking blood pressure, weight, glucose levels or lung function, can contain clues about worsening cognitive function. You may see these clues before doctors do, so an awareness of what changes occur at the beginning of the dementia process is important.12
Comorbid conditions might include coronary heart disease, hypertension, heart failure, stroke/trans ischaemic attacks, diabetes, chronic obstructive pulmonary disease, cancer, painful conditions, depression, schizophrenia or bipolar disorder, neurological disorders and dementia.14 Browne and colleagues15 identified that 90% of people with dementia have at least one comorbid condition with the most frequently seen being hypertension (53%), painful conditions (34%) and depression (24%). Other studies have indicated that 61% of people with dementia have at least three co-morbid conditions.16,17 There is an increased risk of comorbid conditions for people over 65 who have dementia. On average they have four comorbidities as opposed to those in the same age range without dementia who have on average two comorbid conditions.18 Dementia is caused by diseases that affect the brain and so in some cases dementia cannot exist without the presence of a co-morbid condition. This is particularly true of vascular dementia which requires the patient to have a pre-existing vascular disease.
DEMENTIA AND LEARNING DISABILITIES
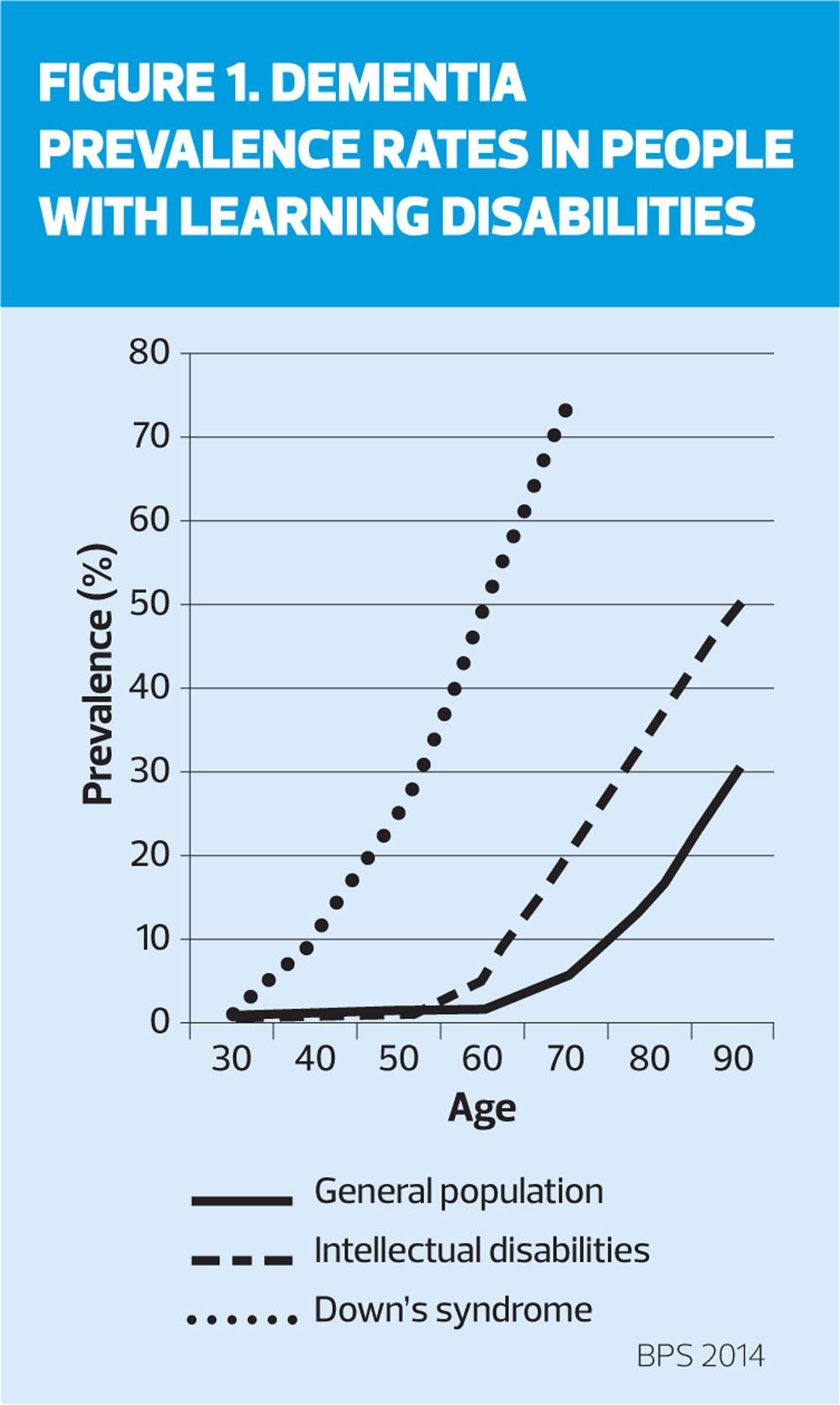
People with learning disabilities are at increased risk of developing dementia at an earlier age than the general population; about 13% incidence in the 60 to 65-year-old age group compared with 1% in the general population (Figure 2).19 People with Down’s syndrome are at particular risk of early onset Alzheimer’s disease.20 The symptoms of dementia in people with Down's syndrome are similar to those seen in the general population, although there are some subtle differences. Changes in behaviour and personality, or loss of the ability to perform activities of daily living are common indicators. However, memory loss, which is usually the most common early symptom of Alzheimer's disease in the general, older population may not be seen so frequently in people with Down’s syndrome.
OPPORTUNITIES
Patients who are living with the afore-mentioned co-morbid conditions and learning disabilities are invited to an annual health review in primary care as part of the expectations laid out in the Quality Outcomes Framework (QOF).21 In Scotland the arrangements differ, with Scottish GP’s offering chronic disease management reviews as part of an enhanced model of care within a differing contractual framework.22
Currently in many areas, as part of the annual health review process for many of the comorbid conditions, the patient is also asked the question: ‘Do you have any concerns about your memory?’ with the response recorded as ‘Yes’ or ‘No’. However, as we have already established, a diagnosis of dementia should not based solely upon concerns about memory. Maximising on these health checks and reviews can be vital to offering people with dementia and their families the opportunity to seek a timely diagnosis, or it may be an opportunity missed. Although general population screening is neither welcomed by clinicians nor validated by research, there is justification to consider screening high risk populations.23
COGNITIVE ASSESSMENT MEASURES
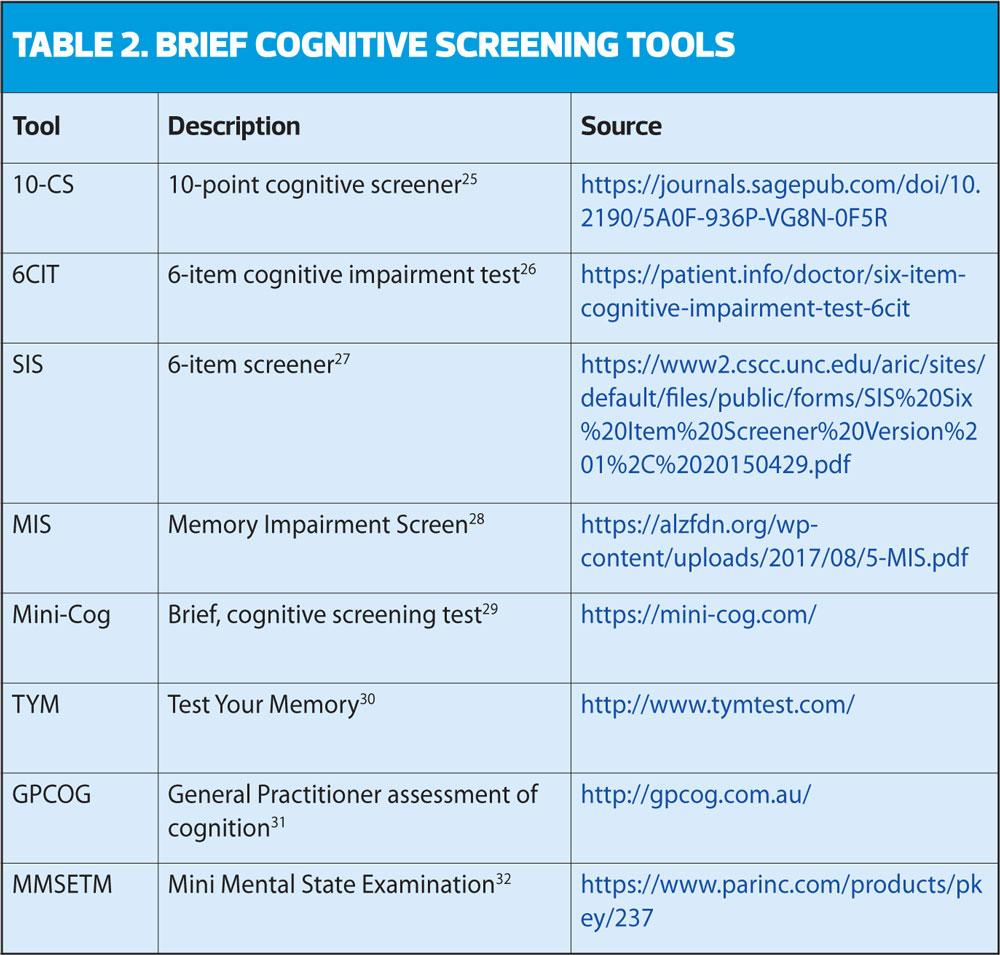
When testing or measuring a person’s cognition, you should use a validated, brief and structured cognitive instrument. This may not be as straight forward as you may think; there are a plethora of brief cognitive assessment tools available that are targeted for administration in primary care, but which is the best one to use? Lorentz et al24 contrasted and compared brief cognitive measures and concluded that there were three tests that showed the most promise for broad application in primary care settings. These were the Mini-Cog, the Memory Impairment Screen, and the General Practitioner Assessment of Cognition (GPCOG). However, there are several others recommended by NICE in the recent dementia guideline update (Table 2).6 The Mini Mental State Examination (MMSE)32 was previously in widespread use but is used less frequently now due to copyright restrictions.
Consent should be sought at all times from patients prior to the administration of any brief cognitive assessment tool; patients have the right to refuse. However, if you believe a patient lacks the capacity to consent and you have assessed their capacity appropriately according to the Mental Capacity Act,33 you may consider its administration to be in their best interests. It should be noted that such screening tools should not be seen as a diagnostic, but should act as an indicator for further investigation. It must also be stressed that if a patient’s result on the brief cognitive assessment tool does not indicate a problem with cognition, yet the patient or someone close to them offers information that would suggest they’re having difficulty this should not be ignored, and further and fuller assessment is recommended.6
Alongside the administration of a brief cognitive assessment it is essential that you have a clear understanding of what actions you need to take if further assessment is required. It is likely that this will vary locally and will be dependent on whether the dementia screening was carried out opportunistically or as part of a planned appointment where the patient has self-presented with concerns about their memory. You should have a clear understanding of local processes and pathways for memory assessment, so you are able to offer patients advice and information on what to expect within the diagnostic pathway. You may indicate the further tests that they may expect, such as, blood tests and urinalysis to exclude other reversible causes of their symptoms and onward referral to memory assessment services, which then may involve a head scan.
It is also important to offer the patient and their family information on where they can access specialist support and information relating to dementia,
e.g. Admiral Nurse Dementia Helpline, Alzheimer’s Society, etc.
CASE STUDY: SLIDING DOORS
Identifying symptoms that lead to a timely diagnosis of dementia can be life-changing for patients, especially as this will lead to appropriate treatment and support and enable them to make plans and decisions about their future wishes and preferences. Left undiagnosed and unsupported dementia can have an insidious and devastating impact on the outcomes for patients and their families as this case study illustrates.
Version A
Mr Brown attends the practice for his annual health review. He has diabetes, COPD, and heart failure. His wife has recently died, and he now lives alone.
As part of the annual review you ask him if he has any concerns about his memory, at which point he smiles and says no. You continue with the review and note that his blood sugar is slightly higher than normal. You discuss his raised blood sugar and offer some dietary advice. He admits he has been snacking a lot since his wife died. You ask whether he needs any help at home; Mr Brown declines and says his daughter helps him and he leaves.
Mr Brown is admitted to hospital three months later following a stroke and hyperglycaemia. He develops delirium while in hospital and it is felt it is not safe for him to return home, so he is discharged to a care home and does not return home.
Version B
Mr Brown attends the practice for his annual health review. He has diabetes, COPD, and heart failure. His wife has recently died, and he now lives alone.
As part of his review you ask Mr Brown if he would be happy to complete a Mini-Cog cognitive assessment, which you describe as a ‘memory test’. He agrees, and you find he has significant difficulty with recall and is unable to draw a clock face. You ask him if he will agree to come back for another appointment accompanied by his daughter Sarah, to explore this further which he agrees to.
At the next appointment you take a full history from Mr Brown and a collaborative history from Sarah, you administer a GPCOG31 with Mr Brown, and Sarah completes the informant interview. The results concur that Mr Brown is experiencing difficulties with recall, processing and in managing his activities of daily living. You also ask Mr Brown about his mood and although he says that he misses his wife and he sometimes feels lonely there are no obvious signs of depression. Sarah advises that her mother had been her father’s carer for some time, preparing all the meals and helping him to manage his medication and she has been concerned about him. This has impacted upon her own wellbeing as she is trying to work and support her father.
With Mr Brown’s consent you request a blood and urine test to rule out any reversible causes of his cognitive decline. There are no abnormalities found and Mr Brown, who has mental capacity, consents to be referred to the local memory assessment services. You also gain his consent to make a referral to social services to explore support options. Social services arrange a package of care to support Mr Brown with his medication, meals and personal care.
Mr Brown receives a diagnosis of vascular dementia. Due to the increased support his diabetes is now better controlled, and he and his daughter attend a dementia support group. Mr Brown continues to live at home with support from the carers and Sarah who is now able to cope better now her father has a diagnosis and appropriate support.
This case illustrates the significance that a dementia diagnosis can have on outcomes for a patient: it can be seen as an important transition for the person with dementia from the uncertainty of the early cognitive and behavioural changes to a phase in which they adjust and learn to live with impairment and loss of function.34 This is important as it is easier for a person with dementia to make adjustments in the earlier stages of their dementia that might enable them to manage other comorbid conditions more effectively. The diagnosis opens the door to post diagnostic support services that can help the person with dementia and their family to come to terms with the condition and gain a better understanding of its effects, enabling them to live better with their condition as it progresses.
Comorbidity among patients with dementia can present real challenges; evidence suggests that the presence of certain co-morbid medical conditions may exacerbate the progression of dementia, but equally the presence of dementia can impact on the ability of patients to engage in and self-manage treatment for other chronic co-morbid conditions.15, 35 Poor management of comorbid conditions can also increase burden on the carers.36
All too frequently, health services and systems are set up for single disease management as opposed to a more holistic approach that considers the multiple health needs of patients and how each impacts on the other. By identifying dementia in the above case scenario, preventative approaches could be implemented that significantly reduced the risk of deterioration, both mental and physical, for Mr Brown and his daughter Sarah. Evidence suggests that people with dementia and comorbid conditions are more likely to have increased health service usage than those without dementia,15 which may increase the incidence of unplanned admissions to hospital. People with dementia often have reduced cognitive and functional reserves, they are left particularly vulnerable to poor outcomes and adverse events if they are admitted to an acute hospital; such as delirium, falls, immobility, incontinence and further functional decline.37,38
As a result, older people with dementia who are admitted to hospital following an acute event have a higher risk of mortality, longer length of stay in hospital and reduced quality of life compared to those without dementia or cognitive impairment,37 and are more likely to be discharged to a care setting rather than return home.38
CONCLUSION
Practice nurses are well placed to be a catalyst for making positive changes in increasing the identification and screening of patients who may have dementia as part of a holistic annual health assessment. This can be as a direct result of their own patient care, through influencing and educating others within the practice, or by changing and enhancing existing processes. The practice nurse may be the only health care professional that the patient comes into contact with on a regular basis and is therefore are well placed in to engage with and identify patients at an earlier stage.
In order to change outcomes for people with dementia and their families there needs to be greater understanding and awareness of the risk factors and prevalence of dementia in relation to other comorbid conditions – not only to support identification but also to improve the long-term management of the symptoms and effects.
REFERENCES
1. Fratiglioni L, Qiu C. Epidemiology of dementia. In: Dening T, Thomas A. (Eds) 2nd edition, Oxford Textbook of Old Age Psychiatry, Oxford: Oxford University Press; 2013.
2. Prince M, Knapp M, Guerchet M, et al. Dementia UK: Second Edition-Overview. Alzheimer’s Society;2014
3. Office for National Statistics Statistical bulletin: Deaths registered in England and Wales (series DR): 2016, 2017 https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/
4. NHS England. The Prime Minister’s Challenge on Dementia 2020.
5. NHS Digital. Dementia: 65+ Estimated diagnosis rate, 2019. https://digital.nhs.uk/data-and-information/national-indicator-library/dementia-65-estimated-diagnosis-rate
6. NICE NG97. Dementia: assessment, management and support for people living with dementia and their carers, 2018 https://www.nice.org.uk/guidance/ng97
7. World Health Organization (WHO) ICD-10: International Statistical Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. 10th edition, Geneva: WHO; 1992
8. American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), Washington CD: American Psychiatric Association; 2013
9. Robinson L,Tang E, Taylor JP. Dementia: timely diagnosis and early intervention. BMJ 2015; 350 :h3029.
10. Arlt S, Hornung J, Eichenlaub M, et al. The patient with dementia, the caregiver and the doctor: cognition, depression and quality of life from three perspectives. International Journal of Geriatric Psychiatry 2008;23:604-610
11. Older people are more scared of dementia than cancer, poll finds. The Telegraph, 04 Aug 2014. https://www.telegraph.co.uk/news/health/elder/11008905/Older-people-are-more-scared-of-dementia-than-cancer-poll-finds.html
12. Iliffe S. (in press) Care of people with dementia in a primary care setting. In: Harrison Dening, K. (Ed) Evidence-Based Practice in Dementia for Nurses and Nursing Students. London: Jessica Kingsley Publishers.
13. Hughes CP, Berg L, Danziger WL, et al. A New Clinical Scale for the staging of Dementia. Br J Psychiatry 1982;140:566-572.
14. Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for healthcare, research and medical education: a cross sectional study.
Lancet. 2012 Jul 7;380(9836):37-43.
15. Browne J, Edwards DA, Rhodes K, et al. Association of Comorbidity and heath service usage among patients with dementia in the UK: a population -based study. BMJ Open 2017;7: e0125456. doi:10.1136/bmjopen-2016-012546
16. Scrutton J, Brancati CU. Dementia and comorbidities: Ensuring parity of care. 2016 https://ilcuk.org.uk/wp-content/uploads/2018/10/Dementia-and-Comorbidities-Ensuring-Parity-of-Care.pdf
17. Timmons S, O’Shea E, O’Neill D, et al. Acute Hospital dementia care: results from a national audit. BMC Geriatrics. 2016;16:113.
18. Poblador-Plou B, Calderon-Larranaga A, Marta-Moreno J, et al. Comorbidity of dementia: a cross-sectional study of primary care older patients. BMC Psychiatry. 2014;14:84. doi:10.1186/1471-244X-14-84.
19. British Psychological Society. Dementia and People with Intellectual disabilities. Guidance on the assessment, diagnosis, interventions and support of people with intellectual disabilities who develop dementia, 2014. https://www1.bps.org.uk/system/files/Public%20files/rep77_dementia_and_id.pdf
20. Public Health England (2018) Guidance. Dementia and people with learning disabilities: making reasonable adjustments guidance, 2018. https://www.gov.uk/government/publications/people-with-dementia-and-learning-disabilities-reasonable-adjustments/dementia-and-people-with-learning-disabilities
21. NHS England. 2018/19 General Medical Services (GMS) contract Quality and Outcomes Framework (QOF) Guidance for GMS contract 2018/19. https://www.nhsemployers.org/-/media/Employers/Documents/Primary-care-contracts/QOF/2018-19/2018-19-QOF-guidance-for-stakeholders.PDF?la=en&hash=6A53571FC0F7A63FA7354951C733B9E6011EC2CD
22. Scottish Government. GMS Contract: 2018. https://www.gov.scot/publications/2018-gms-contract-scotland/pages/5/
23. Barrett E, Burns A. Dementia Revealed. What Primary Care Needs to Know. A Primer for General Practice. Department of Health, 2014. https://www.england.nhs.uk/wp-content/uploads/2014/09/dementia-revealed-toolkit.pdf
24. Lorentz WJ, Scanlan JM, Borson S. Brief Screening Tests for Dementia. Can J Psychiatry 2002;47(8):723–733.
25. Manos PJ, Wu R. The ten-point clock test: a quick screen and grading method for cognitive impairment in medical and surgical patients. Int J Psychiatry Med 1994;24:229-244.
26. Brooke P, Bullock R. Validation of a 6-item cognitive impairment test with a view to primary care usage. Int J Geriatr Psychiatry 1999;14(11):936-40.
27. Callaghan CM, Unverzagt FW, Hui SL, et al. Six-Item Screener to identify cognitive impairment among potential subjects for clinical research. Med Care 2002;40:771–781.
28. Buschke H, Kuslansky G, Katz M, et al. Screening for dementia with the Memory Impairment Screen. Neurology 1999;52(2):231 DOI: 10.1212/WNL.52.2.231
29. Borson S, Scanlan JM, Chen PJ, et al. The Mini-Cog as a screen for dementia: Validation in a population-based sample. J Am Geriatr Soc 2003;51:1451–1454.
30. Brown J, Pengas G, Dawson K, et al. Self-administered cognitive screening test (TYM) for detection of Alzheimer's disease: cross sectional study. BMJ 2009;338:b2030. doi: 10.1136/bmj.b2030.
31. Brodaty H, Pond D, Kemp NM, et al. The GPCOG: a new screening test for dementia designed for general practice. J Am Geriatr Soc 2002;50(3):530-534.
32. Folstein MF, Folstein SE, McHugh PR. (1975). "Mini-mental status". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12 (3): 189–98.
33. Mental Capacity Act 2005. http://www.legislation.gov.uk/ukpga/2005/9/data.pdf
34. Woods RT, Moniz-Cook E, Illiffe S, et al. Dementia: issues in early recognition and intervention in primary care. J Royal Soc Med 2003;96:320-323.
35. Bunn F, Burn AM, Goodman C, et al. Comorbidity and dementia: a mixed-method study on improving health care for people with dementia (CoDem). Health Service Delivery Research. 2016;4(8) 02.2016.p.1
36. Fox C, SmithT, Maidment I, et al. The importance of detecting and managing comorbidities in people with dementia. Age Ageing 2014;43:741-743
37. George J, Long S, Vincent C. How can we keep patients with dementia safe in our acute hospital’s? A review of challenges and solutions. J Royal Soc Med 2013;106(9) 355–361
38. Fogg C, Meredith P, Bridges J, et al. The relationship between cognitive impairment, mortality and discharge characteristics in a large cohort of older adults with unscheduled admissions to an acute hospital: a retrospective observational study. Age Ageing 2017;46: 794–801
Related articles
View all Articles