Dementia and the management of comorbidity
Zena Aldridge
Zena Aldridge
Admiral Nurse Research Fellow,
Dementia UK
Karen Harrison Dening
Head of Research and Publications, Dementia UK
General practice nurses should be aware of issues that may arise when long term conditions and dementia co-exist. Subtle changes to practice can have a significant positive impact for people with dementia, their families and carers
The UK population is ageing. By 2027, more than one-in-five of the population in the UK will be 65 years or older, and within 50 years there are expected to be an additional 8.6 million people over 65.1
The impact of this on healthcare services will be significant as many older people often have complex health and social care needs with most over 65s having at least one long-term condition increasing to two by the age of 75.2 This number increases further with age; 80% of 85-year-old people with a known long-term condition have at least a two further comorbid conditions and 40% have in excess of four comorbid conditions.3
People with dementia are known to have on average three comorbid conditions in addition to their dementia.4 Comorbidities include conditions such as chronic obstructive pulmonary disease (COPD), diabetes, heart failure, hypertension, vascular or heart disease and musculoskeletal disorders.5 The true extent of comorbidities experienced by people with dementia may remain underestimated due to the difficulties they have in communicating and reporting their symptoms.6 So how we can better support a person with dementia who also has another long term condition?
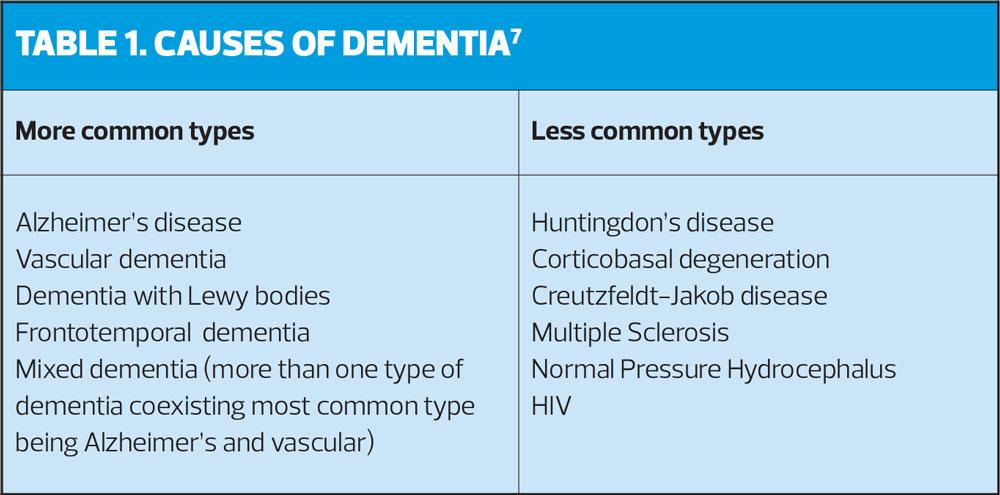
Dementia is an umbrella term used to describe a broad range of symptoms including memory loss, confusion, personality and behavioural changes and difficulty carrying out everyday tasks.7 Dementia is a chronic, life-limiting condition caused by underlying diseases and brain disorders such as Alzheimer’s disease and vascular disease. Dementia and Alzheimer’s disease remained the leading cause of death in England and Wales, accounting for 12.0% of all deaths registered in 2016.8
COMORBIDITY AND MULTIMORBIDITY
Comorbidity is the term used when a person has two or more chronic, long-term conditions such as schizophrenia and diabetes and multimorbidity there are more than two conditions.9 Comorbidity and multimorbidity can present real challenges in primary care as often systems are in place that promote the management and review of singular disease processes rather than considering the impact of one condition on another. This can lead to an effect called diagnostic overshadowing (such as attributing all behaviours to dementia), that is to say that symptoms are attributed to one condition as opposed to being seen as the result of a wider interaction with other comorbidities. It is also important to note that dementia and comorbid conditions interact with each other which can cause either complication with treatment, or an acceleration or exacerbation of one of the individual diseases.6
DIABETES, DEMENTIA AND FRAILTY
Diabetes, dementia and frailty are conditions that are common in the older person and management can be particularly challenging when they co-exist. Diabetes and dementia appear to be linked epidemiologically and share a common biological mechanism.10 The development of dementia in older people with diabetes will have a significant impact on how they continue to manage their pre-existing diabetes and may increase the risk of hypoglycaemia and frailty which can lead to poor outcomes. Similarly, frailty is a state of increased vulnerability resulting from ageing and is associated with a decline in the body’s physical and psychological reserves which will also have an impact on a person’s ability to self-manage disease, and increases the level of disability in diabetes.11
Cognitive impairment and dementia are more common in diabetes and are also associated with an increased risk of hypoglycaemia, poor treatment adherence and increased dependence on others.12
When such conditions co-exist, a multidisciplinary holistic assessment of physical, psychosocial and functional capabilities, including anticipated risks and device management strategies, is recommended. The aim is to maintain functional independence for a long as possible, improve the quality of life, and reduce symptoms and medicine burden.12 In patients with diabetes and dementia, there may need to be a shift in the goals of care as the dementia advances, such as a relaxation of glycaemic targets and stopping or reducing the dose of glucose-lowering drugs.
COPD AND DEMENTIA
Inhaled bronchodilators, with or without inhaled corticosteroids, are the mainstay of therapy in patients with COPD, and treatment regimens may be complex.13 It is worth bearing in mind that the physical and cognitive decline associated with dementia, alongside factors such as hearing or sight loss, can affect patients' ability to adhere to complex treatment regimens.13 Therefore, early detection of cognitive impairment has the potential to positively impact on COPD management and is essential in determining appropriate treatment and support.14
The choice of inhaler device should be guided by the physical and cognitive abilities of each individual patient. Non-adherence in this patient group may not only be due to their forgetting to take medication but can also be influenced by lack of understanding of the rationale for the treatment, apathy or functional impairment due to their dementia, and these can collectively impact on their ability to use their inhaler devices. Where you detect poor symptom control or poor compliance to medications it may be necessary to refer to a specialist for assessment of suitability for long-term nebuliser therapy, as this can be administered with support of others.13 Ultimately, as dementia progresses COPD management may become more difficult and goals of care reduced to basic symptom control rather than preventing disease progression; this approach may be considered less restrictive and in the patient’s best interests.15
IMPROVING TREATMENT ADHERENCE
There are opportunities to improve adherence, but such interventions should be balanced against the patient’s wishes, if they have capacity, and the perceived benefits and outcomes of any treatment.
A full medication review may identify opportunities to reduce the number of medications as well as to coordinate their administration, for example, reducing the administration times from four times a day to twice a day; dispensing tablets in dossette boxes with a prompt mechanism may also be helpful in improving adherence. Teleprompting, if available, may also support improved adherence but of course this does not ameliorate concerns relating to poor administration techniques with injection or inhaler devices.
Assistive technology medicine management devices may also be an option, but they are often introduced reactively as opposed to proactively which may make it more difficult for the person with dementia to adapt to, and learn to use them effectively. It is suggested that although assistive technology may open up conversation and be a route to accessing support, it may have limited impact on maintaining independence and safety for the person living with dementia,16 so a thorough assessment and ongoing review of effectiveness is vital. As dementia progresses more formalised support may be required to prompt and/or administer necessary treatments by district nursing services, formal carers or family carers.
FAMILY CARERS OF PEOPLE WITH DEMENTIA
Two thirds of people with dementia live in the community, many of whom are supported by the 700,000 informal, family carers that provide unpaid care to family or friends living with dementia.17 Poor management of comorbid conditions can place an extra burden on the family carer but this often goes unrecognised. Families and carers should be considered as partners in the care and treatment of people with dementia, alongside the person with dementia within the context of a three-way relationship with healthcare professionals.18 Carers can be a great asset to the practice nurse in supporting the person with dementia to manage comorbid conditions in terms of:
- Remembering health check appointments
- Administering medicines and treatments, and
- Influencing healthier lifestyle choices that may prevent deterioration or exacerbation of long term conditions.
However, carers often receive little information and guidance in this role. Their input, and so potential, often goes unrecognised therefore excluding them from care planning and decision-making processes relating to the person with dementia’s care.19
Carers play a key role in decision-making particularly when the person they care for is in the more advanced stages of dementia at which point the person may have difficulties with communication or reduced mental capacity to make their own decisions. It is important to acknowledge that if a patient lacks mental capacity to make decisions about their treatment carers should be consulted and included in discussions around treatment and care planning within the context of Best Interests decision making. There is a legal obligation to consult with a family carer or named person with a Lasting Power of Attorney for Health and Welfare, which gives them the legal power to make decisions on behalf of the person with dementia about their care and treatment.15
THE IMPORTANCE OF ADVANCE CARE PLANNING
Discussions to enable future planning for care and wishes and preferences in the long-term management of comorbidities are useful for all involved.20 In the UK, the general consensus is that end of life care planning and discussion of future wishes should begin in the early stages of dementia, before the individual with the diagnosis loses capacity.9 A person with dementia, even in the early stages of the illness, may have difficulties considering their preferences for future care.21 However, it is essential to support their autonomy wherever possible and support shared decision-making involving family carers.
Failure to identify dementia and the potential impact it may have on the management and treatment of comorbid conditions can have a detrimental effect for the person living with dementia, their family and the wider health and social care system as the case study illustrates.
It is acknowledged that self-management should be an important component of supporting people with long term conditions, however, there has been very little research into the capability of patients to self-manage particularly when they live with multiple conditions.24 It is vital that open and honest conversations in balancing independence and safety in relation to self-management are carried out in a timely manner.14 There is a lack of research into the impact the presence of dementia has on the treatment and management of accompanying comorbid, long-term conditions. What research there is focuses on the risk factors and relationships that comorbid conditions and their treatment have in relation to developing dementia, rather than when dementia is one of the comorbidities
Implementing opportunistic dementia screening in high risk patients (Box 1) and supporting timely diagnosis of dementia for those with comorbid conditions can better inform treatment plans, identify the correct support needed to manage conditions safely and effectively and reduce risk of crises. Equally, identifying and managing comorbid conditions in patients diagnosed with dementia can improve cognition and functioning and improve the quality of life for the person with dementia and their family carers.
Managing dementia and comorbid conditions requires a person-centred approach to care and treatment, with the patient and their families seen as key partners in care. To facilitate this, nursing staff need to have an increased awareness and understanding of the potential impact dementia has on managing comorbid conditions and vice versa. Treatment needs to be tailored to the care goals, choices and ability of the patient and their family rather than a task focussed single disease approach to managing comorbidities.
CASE STUDY
TWO SIDES OF THE SAME COIN
John is an 82-year-old man who lives with his wife Mary. He has type 1 diabetes, COPD and osteoarthritis. He regularly attends the practice and is today attending for his annual diabetes review. His blood sugars are high when checked, and over the past year Mary has called the paramedics out on three occasions as John had become unresponsive due to hypoglycaemia.
You refer to his notes and see that his insulin dosage has been changed on three occasions over the last 18 months due to the instability of his blood sugars. John advises that he self-manages his insulin as prescribed and has no idea why they are unstable, he states that he has managed his diabetes for the past 50 years without incident. He is worried about what might be causing these changes and how it will affect him if his diabetes cannot be stabilised.
You offer some advice and reassurance and take bloods and request a review with the diabetes specialist nurse. John leaves satisfied with the action plan, however, a week later – two days before his appointment with the diabetes specialist nurse, John is found unresponsive during the night by his wife, he is hypoxic and hypoglycaemic, and paramedics take him to hospital.
THE FLIP SIDE OF THE COIN
John is an 82-year-old man who lives with his wife Mary. He has always self-managed his conditions – type 1 diabetes, COPD and osteoarthritis.
He is unaware that he has not been taking his insulin, inhalers and pain relief as prescribed. He can completely forget to take his medication on some days, and on others he takes more than the prescribed dose as he has forgotten that he has already administered it. Mary has noticed that he has been drowsier and more irritable, and his mood is low of late, but she has attributed that to his age. However, she also suspects that he is getting muddled with his medication and when she raises this with him, he becomes defensive and denies there is a problem. His diet and appetite have always fluctuated and he has managed his insulin accordingly, but over the past 18 months he has not been eating all of his meals as he is often sleeping, and he has experienced three episodes of hypoglycaemia, which have required intervention from paramedics.
Mary has been struggling to sleep over the past 12 months as she is so concerned about the hypoglycaemic episodes and fears that it will happen again, as she is the only one in the house and feels the heavy responsibility of keeping an eye on him. She is struggling to cope, and she often becomes tearful. She knows that he attends the practice regularly so believes that if there were concerns the practice would contact her.
Following his last appointment John tells Mary he is going to see the diabetes specialist nurse; relieved, she suggests she attends too, to which he reluctantly agrees.
CASE STUDY: OUTCOME
John’s admission was attributed to poor management of his diabetes, COPD and pain. Alongside hypoglycaemia and hypoxia, he was found to have high levels of opiates in his system. John remained in hospital for four days and during this time he had an Abbreviated Mental Test Score (AMTS),22 a memory test, which indicated he was showing signs of cognitive impairment. A more comprehensive assessment of his memory following discharge was arranged. Prior to discharge, John’s medication regime was reviewed, and nursing staff arranged for district nurses to administer and monitor his insulin post-discharge and establish the correct dosage. John was given a spacer for his inhalers and staff demonstrated to both John and Mary how to use it appropriately. His pain medication was changed to a transdermal buprenorphine patch to reduce the risk of dosing errors and improve compliance.
Post discharge Mary supported John with his medication administration and district nursing staff taught her how to supervise John, and when it was necessary for her to administer his insulin. Mary was also shown how to administer glucose gel and glucagon should John have another hypoglycaemic episode.
Eight weeks after discharge from hospital John’s cognition was reviewed, although it had improved slightly due to better management of his comorbid conditions and medication, he was still experiencing cognitive difficulties and was referred for a memory assessment. John was diagnosed with a Mixed dementia (Table 1). He and Mary were advised of peer support in his area, via a dementia support group.
John has remained stable for six months with no further hypoglycaemic attacks, he is more alert, and his diet and mood have improved significantly. Mary’s mood and sleep pattern are also much improved and both she and John attend a local dementia support group once a month and have made some new friends whom they also meet outside the group.
CASE STUDY: DISCUSSION
Within the context of the case study the interaction at the GP practice could be seen as completely appropriate; the practice nurse identified that John’s blood sugars were unstable and acted accordingly within the remit of a diabetes review. However, on further examination of the information there were red flags that, if explored further in a more holistic manner, may have uncovered some of the difficulties John and Mary were experiencing sooner enabling preventive action to be taken. John had been attending the practice frequently for reviews of his various conditions, it is arguable that if these reviews were combined, rather than looking at each condition in isolation, the impact one condition places upon another could be highlighted.
CONCLUSION
There is a need to consider the wider implications of comorbidity and its interaction with dementia. This is important in order to balance clinical disease management and symptom control of long term conditions against the quality of life and individual goals of the person with dementia, and their family. To achieve this there need to be structures in place that promote the opportunity to carry out holistic – as opposed to single disease – reviews and a workforce that feels confident and competent to conduct such reviews. There is also a need to improve the recognition of the vital role family carers play in supporting people with dementia to manage their comorbid conditions.
REFERENCES
1.Office of National Statistics (ONS) Overview of the UK population: November 2018 https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/articles/overviewoftheukpopulation/november2018
2.Oliver D, Foot C, Humphries R. Making our health and care systems fit for an ageing population. 2014; London: The King’s Fund. https://www.kingsfund.org.uk/sites/default/files/field/field_publication_file/making-health-care-systems-fit-ageing-population-oliver-foot-humphries-mar14.pdf
3. Age UK. Briefing: Health and Care of Older People in England 2017. https://www.ageuk.org.uk/Documents/EN-GB/For-professionals/Research/The_Health_and_Care_of_Older_People_in_England_2016.pdf?dtrk=true
4. Timmons S, O’Shea E, O’Neill D, et al. Acute hospital dementia care: results from a national audit. BMC Geriatrics 2016;16:113.
5. Bunn F, Burn AM, Goodman C, et al. ( 2016) Co morbidity and dementia: a mixed methods study on improving health care for people with dementia (CoDem): NIHR Journals Library: 2016 Feb. Health Services and Delivery Research 4.8
6. Page A, Beer C, Seubert LJ, et al. Medication use to manage comorbidities for people with dementia : a systematic review. J Pharmacy Pract Res 2018;48:356-367.
7. Sandilyan MB, Dening T. Chapter One: What is Dementia? In: Harrison Dening, K. (Ed) Evidence-based practice in dementia for nurses and nursing students. 2019; London: Jessica Kingsley Publishers.
8. Office for National Statistics (ONS) Statistical bulletin: Deaths registered in England and Wales (series DR): 2016 [Online]. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/
9. NICE NG56. Multimorbidity: clinical assessment and management; 2016. https://www.nice.org.uk/guidance/ng56/chapter/Recommendations
10. Mair ML, Athavale R, Abdelhafiz AH. Practical considerations for managing patients with diabetes and dementia. Expt Rev Endocrinol Metab 2017;12(6):429-440.
11. British Geriatrics Society (BGS). Fit for frailty: consensus best practice guidance for the care of older people living with frailty in community and outpatient settings. 2014; London: British Geriatrics Society. https://www.bgs.org.uk/sites/default/files/content/resources/files/2018-05-23/fff_full.pdf
12. Sinclair AJ, Hillson R, Bayer AJ. National Expert Working Group. Diabetes and dementia in older people: a Best Clinical Practice Statement by a multidisciplinary National Expert Working Group. Diabetic Med 2014;31(9):1024-31.
13. Taffet GG, Donohue JF, Altman PR. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging 2014;9:23-30.
14. Arlt S, Linder R, Rosler A, et al. Adherence to medication in Patients with Dementia Predictors and strategies for improvement. Drugs Aging. 2008; 25(12):1033-1047.
15. Mental Capacity Act (2005). London: HMSO.
16. Bunn F, Goodman C, Reece Jones P, et al. What works for whom in the management of diabetes in people living with dementia: a realist review. BMC Medicine. 2017;15:141.
17. Lewis F, Karlsberg Schaffer S, Sussex J, et al. Trajectory of Dementia in the UK – Making a Difference, 2014; report produced by the Office of Health Economics for Alzheimer’s Research UK. https://www.ohe.org/publications/trajectory-dementia-uk-making-difference
18. Aldridge Z.Chapter 16. Supporting Families and Carers of People with Dementia.?In: Harrison Dening, K.(Ed) Evidence- Base Practice?In: Dementia for Nurses and Nursing Students pgs. 2019; Jessica Kingsley. London.
19. Bunn F, Goodman C, Burn AM. Multimorbidity and frailty in people with dementia. Nursing Standard. 2015; 2;30(1):45-50.
20. Houttekier D, Cohen J, Bilsen J, et al. Place of death of older persons with dementia. A study in five European countries. J Am Geriatr Soc 2010;58(4):751–6.
21. Harrison Dening K, Greenish W, Jones L, et al. Barriers to providing end-of-life care for people with dementia: a whole-system qualitative study. BMJ Support Palliat Care 2012;2(2):103–7.
22. Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age and Ageing 1972;1(4):233-8 http://ageing.oxfordjournals.org/cgi/reprint/1/4/233
23. Valderas JM, Starfield B, Sibbald B, et al. Defining comorbidity: implications for understanding health and health services. Ann Fam Med 2009;7(4):357–63.
24. Barker I, Steventon A, Williamson R, et al. Self-management capability in patients with long-term conditions is associated with reduced healthcare utilisation across a whole health economy: cross-sectional analysis of electronic health records. BMJ Quality and Safety. 2017;27(12): doi:10.1136/bmjqs-2017-007635.
Related articles
View all Articles