Seasonal flu campaign grows in complexity
MANDY GALLOWAY
MANDY GALLOWAY
Editor, Practice Nurse
September sees the start of the annual bunfight that is the seasonal flu vaccination campaign, so here is a reminder about some of the changes and why they have been made
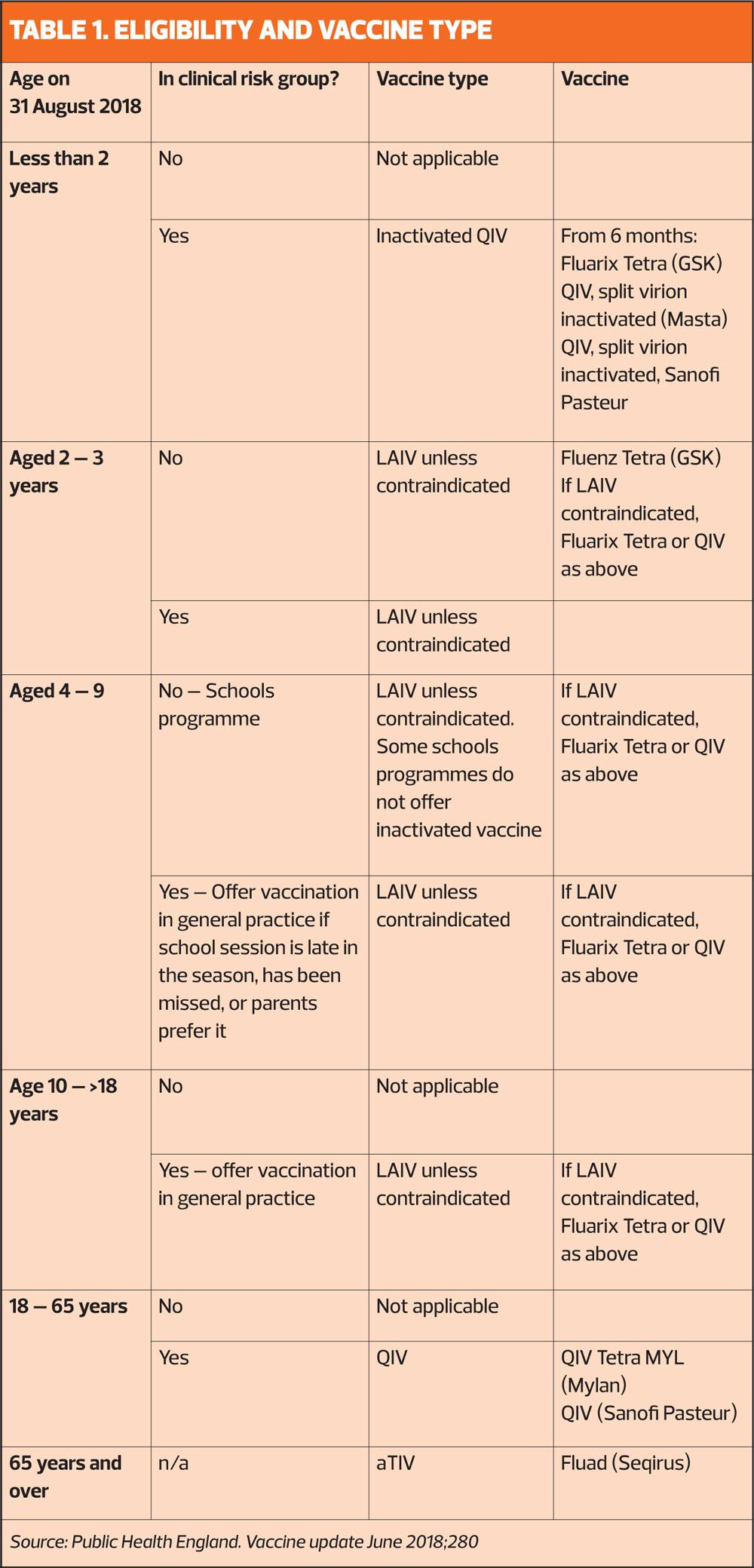
General practice nurses face a far more complicated schedule of flu vaccines for the coming season than at any time since the seasonal flu vaccination campaign was introduced.
NHS England says this should not pose any particular difficulties because practice will be used to handling more than one flu vaccine – for example, live vaccine for most children but inactivated vaccine for those with egg allergies or who are immunocompromised. Nonetheless, the usual procedures for safe administration of vaccine should be observed.
This winter, over 65s should be offered the most recently licensed vaccine – the adjuvant trivalent inactivated vaccine (aTIV), which was licensed in late 2017 and will be available for the first whole flu season this year. Meanwhile, those in the clinical risk groups should be offered quadrivalent influenza vaccine (QIV), which offers protection against two strains of influenza B rather than one.
The childhood flu immunisation programme will, this winter, be delivered predominantly in schools – apart from 2- and 3-year-olds, who will continue to be vaccinated in general practice. The vaccine for this group, as in previous years is the live attenuated influenza vaccine (LAIV) – but this is not licensed for children under 2 years, so any children aged 6 months to 2 years in a clinical risk group should be offered an inactivated quadrivalent vaccine.
A further complication is that for the third year, patients will be able to obtain their vaccination in pharmacies – which may be convenient for some patients, but may also add to possible confusion about who has been vaccinated and whether they have received the most appropriate vaccine for their age and risk status.
Much of the information circulated by Public Health England and NHS England is sent out in February and March, just as the previous flu season is coming to an end. Hopefully practices will have got to grips with what needed to be ordered in advance – but Practice Nurse thought it might be useful to provide a reminder as GPNs gear up for the start of the campaign next month (See Table 1).
WHY HAS A NEW VACCINE BEEN INTRODUCED FOR OVER 65s?
The adjuvanted trivalent inactivated vaccine (aTIV) Fluad® has been licensed in some countries in Europe since 1997, and in the USA since 2015. aTIV has higher immunogenicity in older people, particularly in the over-65s. The priority group for its use is the over-75s for whom the conventional, non-adjuvanted vaccine provides little benefit.1
As people grow older, their immune response declines, a process known as immunosensescence.2 Between 2004 and 2015, the conventional inactivated flu vaccine showed no significant efficacy in the elderly against the A (H3N2) influenza virus. This sub-type of the virus is associated with outbreaks even in highly-vaccinated populations, such as the residents of care homes, and typically results in excess mortality.3
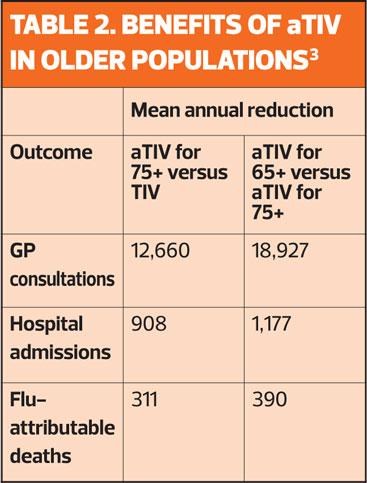
However, the MF59C adjuvant in aTIV has been shown to help the body’s immune system to recognise the inactivated virus particles in the vaccine, which in turn leads to a higher immune response.2 (Table 2)
As the over-65s are disproportionately at risk of hospitalisation for flu-related complications, NHS England has accepted the JCVI recommendation that aTIV will be cost-effective.
Even taking into consideration the anticipated benefits of QIV, aTIV is a more appropriate choice than standard QIV for older people.
It is anticipated that there will be a ‘plentiful’ supply of flu vaccine this season, but the manufacturers of aTIV will be staggering supply over the months of September, October and November.
For this year only, the manufacturer will provide most of the supply of aTIV in pre-filled syringes with a separate needle that will require attachment prior to administration. For next year, the vaccine will be supplied as a pre-filled syringe with an attached needle.
The vaccine can be ordered in packs of 1 and 10, but a limited amount of the single packs will be available this season, so practices are restricted in the amounts of aTIV they can order, in line with their patient lists. The initial priority for aTIV should be those aged 75 years and older.
WHY USE A QUADRIVALENT VACCINE FOR AT RISK GROUPS?
QIV covers the two main influenza B strains and is designed to improve the breadth of protection in seasons when the circulating B strain is not well matched to the single strain contained in the conventional trivalent vaccine. Influenza B strains are more common in children, and the childhood vaccine has been quadrivalent since the programme started.
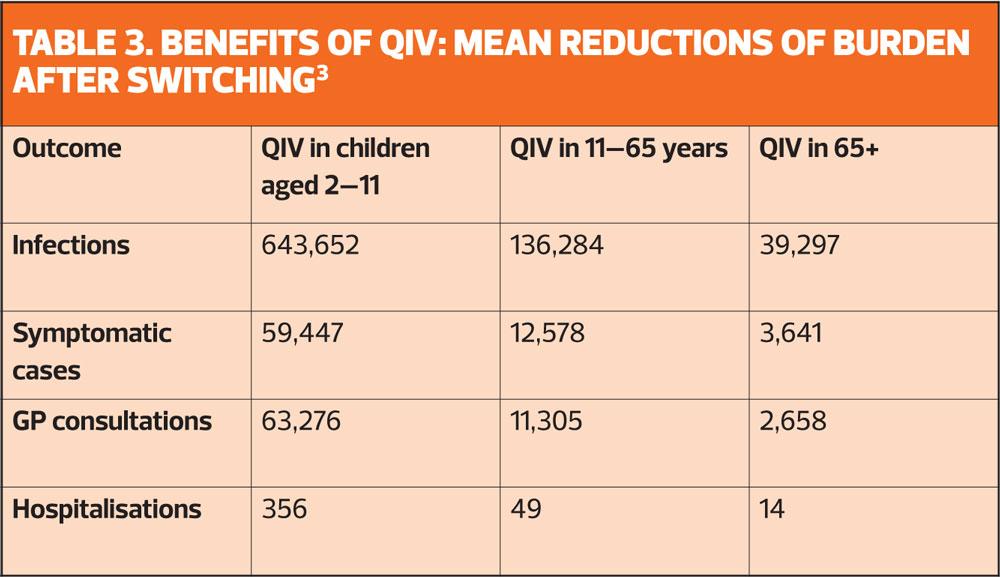
But it is now thought that there will be additional benefits in using QIV for adults and pregnant women who are most at risk of the complications of flu. As shown in Table 3, the benefits in terms of reducing infections, GP consultations and hospital admissions decline in older patient groups. But although the additional health benefits are relatively small, several studies indicate that QIV is likely to be cost-effective if used in at risk groups and pregnant women.3
It is also suggested that healthcare workers might benefit from QIV, especially in years when there is a poor match between the TIV vaccine and the circulating B strain.3
However, the main advantage of QIV is in children, where quadrivalent LAIV offers indirect protection at population level by reducing transmission.3
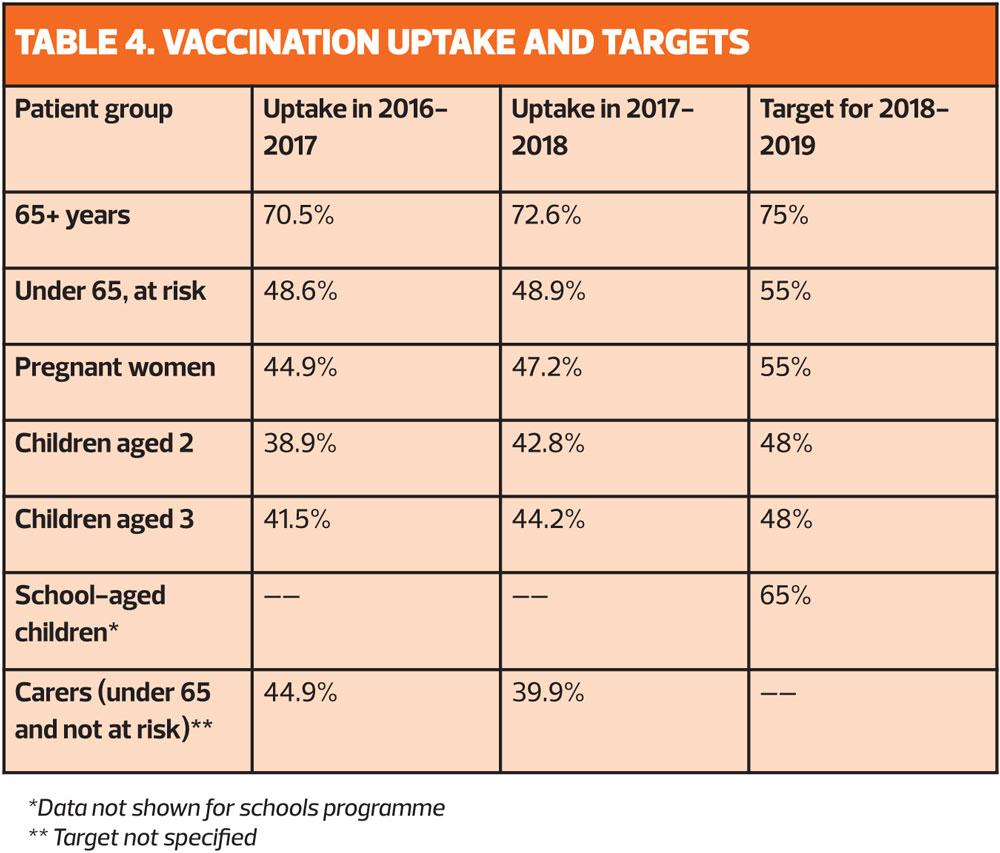
Last season saw record flu vaccination levels, with more than 1.5 million more people getting vaccinated than the previous season. However, NHS England says: ‘We should strive to further improve vaccine uptake in all eligible cohorts.’
Although the enhanced service specification for flu includes payment for vaccines given until 31 March 2018, practices are being urged to start giving vaccination as soon as possible, before flu starts to circulate, and aim to have completed vaccinations by the end of November as the immune response takes about two weeks to develop fully. However, it is still appropriate to offer vaccination to eligible patients at any point in the season, even if patients present late. This is particularly important if it is a late flu season, or for patients newly joining an at risk group, or who become pregnant.
THIS SEASON’S VACCINE
The World Health Organization has recommended the following components for the quadrivalent vaccine:
- For H1N1, an A/Michigan/45/2015-like virus
- For H3N2, an A/Singapore/INFIMH-16-0019/2016-like virus
- For B Victoria, a B/Colorado/06/2017-like virus
- For B Yamagata, a B/Phuket/3073/2013-like virus
For trivalent vaccines, the WHO has recommended inclusion of the Victoria lineage B influenza strain.4
In recent years vaccine effectiveness has been around 50%, but this has ranged from 25% to 70%. Last year, the influenza B/Yamagata lineage was in circulation, which was contained in the quadrivalent vaccine but not in the more widely used trivalent flu vaccine.5
A US study has predicted that this year’s vaccine will have the ‘same reduced efficacy against the dominant circulating strain of influenza A as the vaccine given in 2016 and 2017’ arising from problems in the manufacture of vaccine in eggs.6 However, studies comparing cell-based vaccine production and egg-production are lacking. Earlier this year, the WHO changed its recommendations on two of the components for the 2018 vaccine, but this will not impact on persistent ‘egg-adaptation’ problems.7
CONCLUSION
With more vaccines than ever, GPNs will need to be on the ball to ensure that they have the right vaccines in stock, and administer the right one to the right patient. Last year’s uptake was a credit to general practice teams – everyone from the staff who organise the invitations to the nurse who administers the vaccine and chivvies non-attenders to get vaccinated. But there is still more to be done before we can be confident of meeting the WHO target of 75% across the board. Finally, the most important people to get vaccinated are you, yourselves, to protect yourself, your colleagues, your families and your more vulnerable patients.8
REFERENCES
1. The Green Book Chapter 19: Influenza https://assets.publishing.service.gov.uk/government/uploads/
system/uploads/attachment_data/file/663694/Greenbook_chapter_19_Influenza_.pdf
2. Black S. Safety and effectiveness of MF-59 adjuvant influenza vaccines in children and adults. Vaccine 2015;33(Suppl2):B3-5
3. Public Health England. Summary of data to support the choice of influenza vaccine for adults in primary care, 2018.
4. WHO. Recommended composition of influenza virus vaccines for use in the 2018-19 northern hemisphere influenza season. http://www.who.int/influenza/vaccines/virus/recommendations/2018_19_north/en/
5. Public Health England. Flu and flu vaccines: Expert interview, January 2018. https://publichealthmatters.blog.gov.uk/2018/01/11/flu-and-flu-vaccines-expert-interview/
6. Science Daily. Study predicts 2018 flu vaccine will likely have 20 percent efficacy, April 2018. https://www.sciencedaily.com/releases/2018/04/180419131015.htm
7. Center for Infectious Disease Research and Policy (CIDRAP). WHO changes 2 strains for 2018-19 flu vaccine, 2018. http://www.cidrap.umn.edu/news-perspective/2018/02/who-changes-2-strains-2018-19-flu-vaccine
8. NMC. The Code: Professional standards of practice and behaviour for nurses and midwives, 2015. https://www.nmc.org.uk/globalassets/sitedocuments/nmc-publications/nmc-code.pdf
Related articles
View all Articles