Planning for winter flu immunisation campaign
As practices gear up for this year’s flu vaccination campaign, Practice Nurse summarises what you need to do to hit targets
Last year’s winter flu immunisation campaign was undermined by reports that the vaccine was not effective against some of the strains of flu that entered circulation but flu remains a key factor in NHS winter pressures, and practices have been urged to start preparations for the 2015-16 campaign now.
Early vaccine effectiveness data found that the overall flu vaccine programme for 2014-15 provided low levels of protection against one of the flu subtypes, H3N2 – only 3% overall, compared with 50% typically. This was due to a ‘drifted’ strain that emerged after the vaccine components were selected by the World Health Organization in February 2014.
Public Health England (PHE) says: ‘Flu is unpredictable. It is not possible for the WHO to predict fully the strains that will circulate in any given season, and there is always a risk of a drift and subsequent mismatch. However, this occurs rarely. In nine out of the last ten seasons, the vaccine has provided good to moderate protection against the circulating strains.’
PHE adds that it is ‘crucial’ that last year’s mismatch doesn’t deter people in eligible groups from having the flu vaccination this year. Vaccine remains the best protection against flu, a mismatch between the vaccine and the strains of circulating flu is rare, and the annual immunisation programme is a vital part of the strategy to reduce unplanned hospital admissions and pressure on A&E.
THIS YEAR’S VACCINE
For the 2015-16 flu season, the WHO has recommended that trivalent vaccines should contain:
- An A/California/7/2009 (H1N1)pmd09-like virus
- An A/Switzerland/9715293/2013 (H3N2)-like virus
- A B/Phuket/3073/2013-like virus
In addition, quadrivalent vaccines should also contain a B/Brisbane/60/2008-like virus.
AMBITION
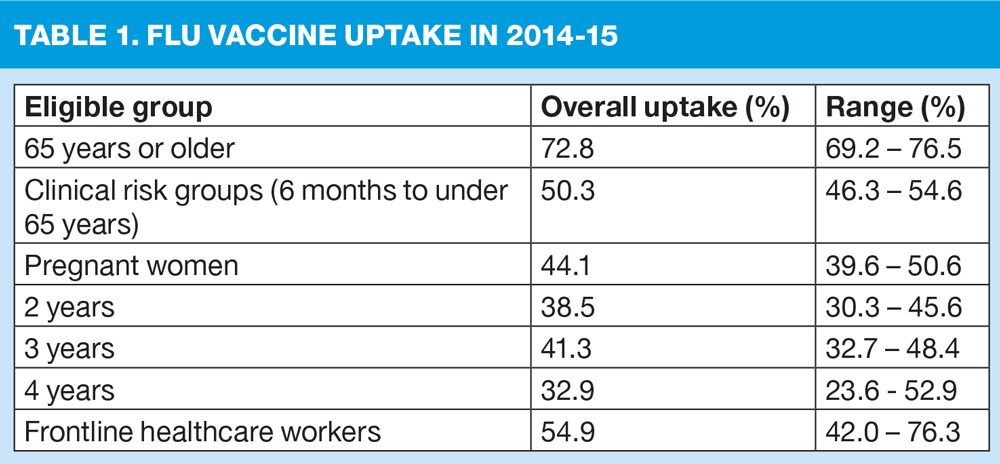
The Government’s target for uptake is – as it has been in previous years – at least 75%. As shown in Table 1, uptake last year fell well below this target, although PHE nonetheless thanked everyone ‘for their hard work’. ‘Vaccine uptake needs to improve,’ said PHE, ‘particularly in those who are at the highest risk of severe disease and mortality from flu but have low rates of vaccine uptake, including those with chronic liver and neurological disease, and people with learning disabilities.’
Children
Every eligible child should be offered the vaccine, and practices will be required to provide evidence that the offer has been made. As a bare minimum, PHE expects uptake levels of between 40% and 60%.
Results from the first year of primary school pilot sites in 2013-14 were ‘encouraging’, PHE says, with reduced numbers of GP attendances for flu-like illnessness and reduced emergency department attendances for respiratory illness in all ages in pilot areas compared with non-pilot areas.
- GP consultation rate for flu-like illness in pilot areas was 17.7/100,000 population compared with 64.5/100,000 in non-pilot areas
- Laboratory confirmed flu in pilot areas was 8.5% compared with 16.2% in non-pilot areas
It is predicted that once the children’s programme is fully implemented, it will prevent many cases of severe flu and flu-related deaths in older adults and people in the clinical risk groups. The main changes to the children’s programme are that this year, children who took part in the primary school pilots last year will be eligible for vaccination in general practice, and children in school years 1 and 2 age will also be offered flu vaccination. Delivery models may vary by area, and although it is likely to be implemented mainly through schools, in some instances children of school years 1 and 2 age will be immunised in primary care.
Unless contraindicated, children aged 24 months to 18 years should be offered the quadrivalent, live attenuated influenza vaccine (LAIV) Fluenz Tetra, which is administered intranasally. Fluarix Tetra, the quadrivalent inactivated vaccine is not licensed for use in children under age 3 years. For younger children requiring an alternative to Fluenz Tetra, there are other split virion, inactivated virus vaccines available via ImmForm. Check the indications in the summary of product characteristics for individual products, and look out for an update to the influenza chapter of the Green Book.
Frontline staff
For frontline staff – including practice nurses – there should be a ‘100% offer’ of vaccination. Health and social care workers have a ‘duty of care’ to protect their patients from infection, and while it remains voluntary, PHE urges all staff to have the vaccine not only to protect their patients, but also their families. Vaccination has been shown to reduce the level of sickness absences, another important factor in managing winter pressures in general, and could make a huge difference to how practices cope with their workload over the winter.
Pregnant women
All pregnant women are recommended to have the flu vaccine, irrespective of their stage of pregnancy. Inactivated vaccine can be administered safely during any trimester, and there has been no study to date that shows any increase in the risk of harm to the unborn baby or in maternal complications.
On the contrary, flu vaccination during pregnancy may reduce the likelihood of preterm birth and smaller infant size at birth, and it also offers some protection to the baby in its first few months of life.
Ideally, vaccine should be given before flu starts to circulate, but it is important that vaccination is offered to any woman who becomes pregnant during the flu season. The perfect time for this to take place is at the woman’s first midwife appointment, but not every midwife is an independent prescriber, so practice nurses should check that vaccine has been given. Local NHS England teams are looking at ways of linking midwifery services with GP practices.
Obesity
Morbidly obese people, i.e. those with a BMI of 40+ may also benefit from a flu vaccination, according to the Joint Committee on Vaccination and Immunisation. Many of these people will already be eligible because they fall within another risk category, but if they do not, practitioners need to use their clinical judgement to decide whether to vaccinate them. They won’t attract a payment under the Direct Enhanced Services (DES) scheme this year, but they may be included in the flu programme in 2016-17.
Related articles
View all Articles