Influenza vaccine technologies: an update
James Wheeldon
James Wheeldon
James Parker
Simon Oakley
Medical Affairs, Sanofi Pasteur UK.
Practice Nurse 2020;50(8):20-24
The pace of change for vaccine manufacturing processes has never been faster. As seasonal infectious pathogens continue to evolve and adapt, so must we in our efforts to safeguard the health of the public. In this review, we look at how influenza vaccines have developed over the years and look to the future
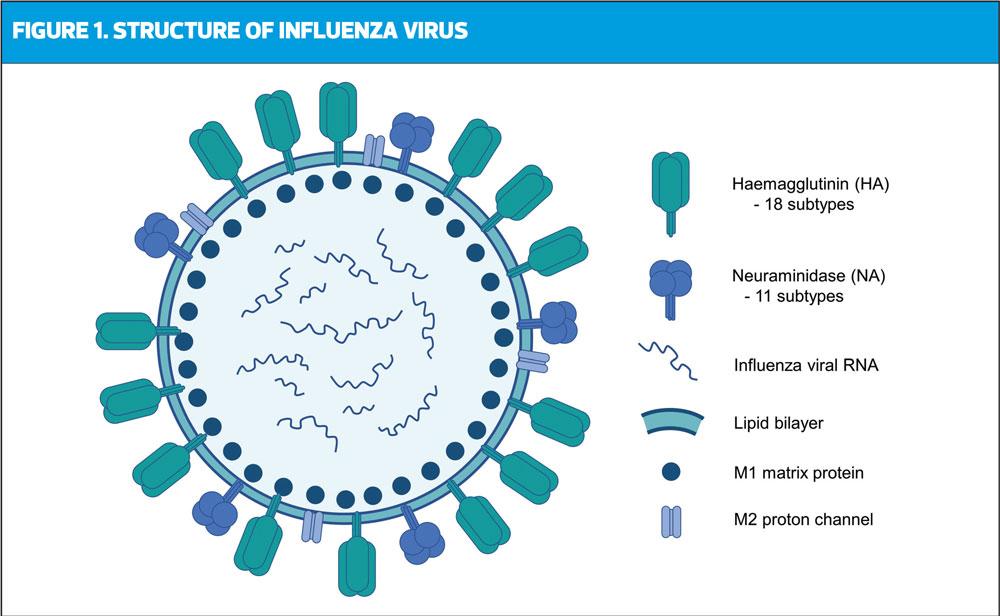
Influenza is a member of the Orthomyxoviridae viral family and is represented by the subtypes A, B, C and D.1,2 Influenza A and B are known to circulate and cause seasonal epidemics in humans; influenza C is rarely detected, and influenza D is not known to cause disease in humans.1 Seasonal circulation is often highly variable, with different countries experiencing different strain prevalence each year. Currently influenza A (H1N1 and H3N2) and B (Victoria and Yamagata) are in circulation within the human population.3
Infections are typically passed on through droplet transmission and the disease presents as an acute respiratory illness, typically with symptoms including sudden fever above 38°C, myalgia (muscle aches/weakness), a dry cough and sore throat.4 While the majority of infections in the healthy population are self-limiting and typically resolve within a week, influenza-like illness (ILI) can cause severe complications in the very young, the elderly and at-risk groups as defined in the Green Book. Annual vaccination is therefore strongly encouraged in eligible groups to reduce the impact on public health and provide herd protection by meeting the 75% vaccine uptake target set by NHS England.5,6
The true burden of disease caused by seasonal influenza is likely to be significantly underestimated.7 Globally, influenza is thought to claim between 290,000-650,000 lives each year.8 In the UK, seasonal influenza infection is estimated to be responsible for 19,000-31,200 hospitalisations and 18,500-24,800 deaths each year.9
Vaccination is the most effective means of protection against influenza infection.1 However, clinicians, researchers and manufacturers are all faced with the same complex challenge to address the high variability and rapid mutation rate of influenza. In this review, we aim to provide an overview of influenza vaccine technology, explore evolving vaccine technologies and understand the impact of these changes on clinical practice.
BRIEF HISTORY OF THE INFLUENZA VACCINE
Ahead of each annual influenza season, the World Health Organization (WHO) issues recommendations to vaccine manufacturers detailing the antigenic composition of the predicted circulating influenza strains, termed candidate vaccine viruses (CVV).10 Manufacturers are then responsible for using the CVVs to produce and supply influenza doses within a 6-9 month timeframe after receiving notification. In 2015 alone, 6.4 billion doses of influenza vaccine were produced globally,11 highlighting the substantial demands manufacturers are facing, in addition to the need for new scalable technologies to ensure timely supply.
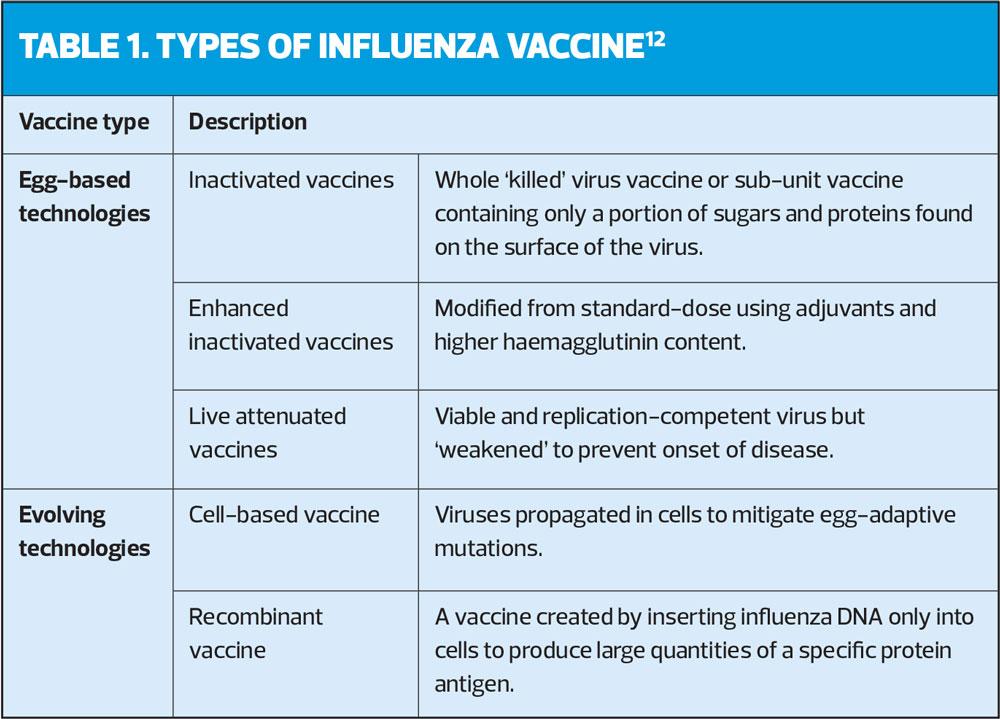
Most conventional influenza vaccine technologies fall under three major categories based on their design (Table 1).12
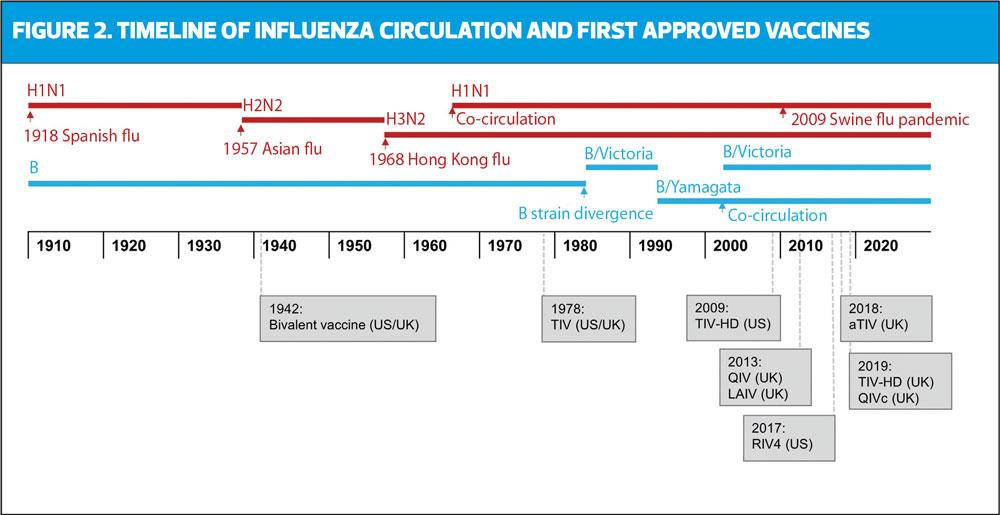
Historically, influenza A strains circulated alternately in humans, and it was not until 1940 that Influenza B was discovered and a bivalent vaccine was developed (Figure 2).13 By 1970, the B strain lineage diverged into two lineages (B/Victoria and B/Yamagata), further impacting the accuracy of vaccine matching. Following the re-emergence and co-circulation of A/H1N1 with A/H3N2 in 1978,14 the first trivalent influenza vaccine (TIV) was developed, containing two haemagglutinin (HA) antigens derived from each of the influenza A strains and one influenza B strain.15 The co-circulation of both B strains occurred in the early 2000s when the B/Victoria lineage reappeared for the first time in 10 years.16 Since TIV may not offer effective cross-protection for the alternate circulating B strain, there was a potential for suboptimal protection during mismatched seasons. This was particularly evident in the 2017/18 season, where UK influenza-associated deaths almost tripled due to the early circulation of influenza in the community, and TIV mismatch against B/Yamagata.17,18 As a result, a quadrivalent influenza vaccine (QIV) was introduced to protect against both B lineages, and is now widely offered to eligible groups in the UK from ages 6 months onwards.19
EGG-BASED TECHNOLOGIES
Inactivated influenza vaccines
Since 1936, fertilised hen eggs have been used in the production of seasonal influenza vaccines and still remain a key component in many vaccine manufacturing processes today.20 In egg-based vaccine production, CVVs for each strain are injected into eggs, incubated over several days to propagate the virus and tested for viral titre, infectivity, specificity and sterility.20 Viable candidate viruses are known as ‘monovalent seed virus’ and are subsequently inactivated (‘killed’), purified, concentrated and formulated into a final vaccine dose.20
Enhanced inactivated influenza vaccines
It is recognised that in certain populations – particularly the elderly and those with compromised immune systems – many vaccines, including influenza, can become less effective. Therefore, a number of approaches have been employed in attempts to mitigate this.
One way to enhance the immunogenicity of a vaccine is by using adjuvants. Adjuvants are excipients that provide additional signals to immune cells to improve antigen recognition, cell activation and promote induction of the humoral response.21 Vaccine adjuvant research began in the 1920s, with development of oil-in-water emulsion and aluminium salt (alum) adjuvants for vaccines against pertussis, tetanus and diphtheria.22 Current adjuvanted influenza vaccines contain the oil-based MF59 adjuvant, an emulsion of naturally occurring squalene oils designed to induce robust immune reactions while being readily metabolised.23 MF59-adjuvanted TIV (aTIV) was first introduced to the UK national influenza vaccination programme for the 2018/19 season for all adults aged ≥65 years and remains as the recommended vaccine for those aged ≥65 years for the 2020/21 season.19,24
Inactivated influenza vaccines can also be enhanced by increasing the dosage. Standard dose TIV (TIV-SD) was previously shown to be sub-optimal in patients ≥65 years compared with healthy young adults.25 To improve immune protection in this population, a high dose trivalent vaccine (TIV-HD) was developed and approved for use in the UK in the ≥65s in 2019. TIV-HD contains a 4-fold increase in HA antigen content, which can improve antibody responses and vaccine effectiveness compared with TIV-SD.26–30
Live attenuated influenza vaccine (LAIV)
Much like conventional inactivated influenza vaccines, LAIVs are propagated in egg and have a multivalent composition.31 In contrast to inactivated influenza vaccines, LAIVs contain viable influenza viruses which have been attenuated (‘weakened’) to mimic natural infection without manifesting into influenza-like illness. LAIV viruses need to ‘infect to protect’ by replicating in host cells and shedding new virus to stimulate an immune response. Given that LAIV virus are attenuated yet still viable, there is a small theoretical risk of transmission. However, it should be noted that the overall risk is considered negligible and, in the US where LAIVs are widely used, this type of vaccine virus transmission is yet to be reported.
Vaccines which mimic natural infection elicit broad humoral and cellular immune responses.32 As an intranasal vaccine, LAIV targets the mucosal surfaces of the upper respiratory tract; in addition to the induction of a serum IgG response, LAIV can also stimulate production of secretory IgA in the nasal and oral cavities, which may be a key factor in immune protection at sites of infection.32
The current evidence suggests that LAIVs are more effective in children, whereas inactivated influenza vaccines tend to be more immunogenic in adults.33 This may partly be explained by observations that LAIV can only induce influenza-specific T-cell responses in children, and not in adults who have accrued extensive pre-existing immunity through previous exposure.34,35
Since children are widespread transmitters of influenza within the community, the rationale for a childhood influenza immunisation programme using a well-accepted, non-invasive vaccine is clear. Based on these data, LAIVs were introduced in the UK for the 2013/14 season and are recommended in the 2020/21 season, as part of the childhood vaccination programme and in selected cohorts, unless contraindicated.19,36
EVOLVING TECHNOLOGIES
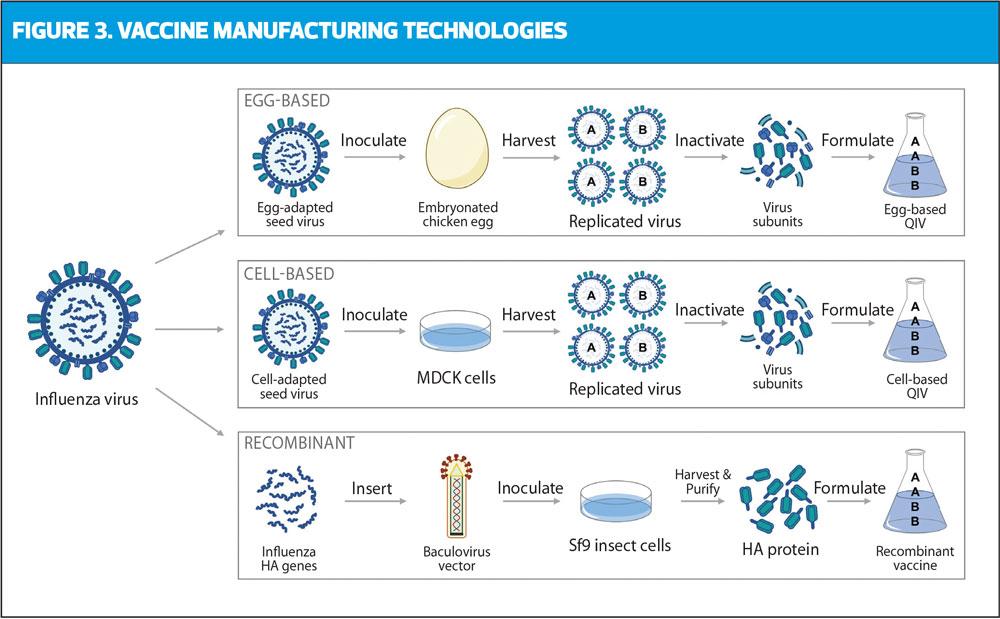
In recent years, a phenomenon known as ‘egg adaptation’ has been recognised. In essence, this is where changes occur to the vaccine virus during the egg-based production process, potentially resulting in a mismatch between the vaccine strain being produced and the circulating wild-type virus. Therefore, alternative methods have been developed to address these potential changes, using non-egg based cell-based and recombinant systems (Figure 3).
Cell-based vaccines
In recent years, manufacturers have been transitioning away from egg-based antigen production in preference for using cell cultures. In cell-based manufacturing, influenza viruses are isolated and grown in vitro in Madin-Darby Canine Kidney (MDCK) cells, rather than fertilised eggs.37 Generally, cell-based vaccines offer several potential advantages over traditional egg-based technologies. Firstly, the use of MDCK cells eliminates egg-adaptive mutations, resulting in CVVs that closely match the circulating strain.38 In practice, this may lead to a greater relative vaccine effectiveness (rVE) for cell-based vaccines compared with egg-based vaccines, as suggested in two studies during the 2017/18 season in the US.39,40 However, another study reported that there was no significant difference to rVE between the two vaccine types.41 There have also been cases published where cell-based systems may be susceptible to passage-mutations, although to a lesser extent than egg-based systems.42,43 Despite this, cell-based manufacturing can mitigate the logistical issues associated with the egg supply from ‘clean’ pathogen-free flocks of hens. MDCK cells can be frozen in large quantities and if manufacturing requires a rapid start, for example in the event of a pandemic, cell-based manufacture can be initiated at relatively short notice.44
Although authorised by the European Medical Agency in 2007, the 2019/20 season marked the first use of cell-based quadrivalent influenza vaccine (QIVc) in the UK for adults aged18-64 in at-risk groups and all over 65s where aTIV is unavailable, and this has continued into the 2020/21 season.5,19
Recombinant vaccines
Recombinant vaccines rely on vector-based technology. Influenza virus genetic material encoding the haemagglutinin (HA) component from the CVV are identified and inserted into plasmid DNA. This is then spliced into a baculovirus expression system, which contains the instructions to produce the target HA proteins. The recombinant baculovirus is used to inoculate host insect cells, which rapidly produce large quantities of influenza HA proteins that are recognised by the host immune system and can elicit a highly specific immune response. The HA proteins are then harvested and purified for recombinant vaccine manufacture. Overall this process yields recombinant HA proteins that are genetically identical to the selected influenza strains without extraneous egg proteins, formaldehyde, antibiotics, or preservatives.
Results from randomised controlled trials (RCTs) and real-world studies indicate that recombinant vaccines have the potential for vaccine efficacy and effectiveness, particularly in times when circulating strains are mismatched in relation to those initially selected by the WHO. This was evident during the 2017/18 season when considerable antigenic mismatch of the circulating strain and the vaccine strains was reported.43 These findings are consistent with data suggesting that recombinant vaccines provide a broad spectrum of antibody protection,45-47 however, the exact biological mechanisms behind this are still unclear.
CONCLUSION
Egg-based manufacturing has long been the foundation of vaccine production. However, the movement toward an in vitro platform is an attractive approach when faced with mismatches and in preparation for pandemic responses, where high volumes of vaccines are required in short time-frames and without the limitation of egg supply. Since mismatches can occur as a result of antigenic mutations in the circulating influenza strains or through egg-adaptation during manufacture, evolving technologies have a critical role to play, to help ensure we stay one step ahead when it comes to vaccine production.
DECLARATION OF INTEREST
The authors are employees of Sanofi Pasteur, which manufactures influenza vaccines.
REFERENCES
1. World Health Organization. Influenza (Seasonal); 2018 https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal)
2. Centers for Disease Control. Pink Book Chapter 12: Influenza; 2019 https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/flu.pdf.
3. World Health Organization. Recommended composition of influenza virus vaccines for use in the 2020-2021 northern hemisphere influenza season; 2020 https://www.who.int/influenza/vaccines/virus/recommendations/202002_recommendation.pdf?ua=1.
4. Public Health England. Green Book Chapter 19: Influenza; 2019.
5. NHS England. The national flu immunisation programme 2019/20; 2019. https://www.england.nhs.uk/wp-content/uploads/2019/03/annual-national-flu-programme-2019-to-2020-1.pdf.
6. Oakley S, Bouchet J, Costello P. Flu vaccination uptake in the UK in at-risk groups: review. Practice Nurse 2019;49(9):12–16.
7. Iuliano A, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018;391(10127):1285–1300.
8. World Health Organization. WHO Influenza Mortality Estimate based on Respiratory Diseases; 2020 https://www.who.int/influenza/surveillance_monitoring/bod/FAQsInfluenzaMortalityEstimate.pdf?ua=1.
9. Pitman RJ, Melegaro A, Gelb D, et al. Assessing the burden of influenza and other respiratory infections in England and Wales. J Infect 2007;54(6):530–8. doi: 10.1016/j.jinf.2006.09.017.
10. World Health Organization. Available candidate vaccine viruses and potency testing reagents; 2020. https://www.who.int/influenza/vaccines/virus/candidates_reagents/home/en/.
11. McLean KA, Goldin S, Nannei C, et al. The 2015 global production capacity of seasonal and pandemic influenza vaccine. Vaccine 2016; doi: 10.1016/j.vaccine.2016.08.019.
12. Oxford Vaccine Group. Types of vaccine; 2019. https://vk.ovg.ox.ac.uk/vk/types-of-vaccine.
13. Hannoun C. The evolving history of influenza viruses and influenza vaccines. Exp Rev Vaccines 2013; doi: 10.1586/14760584.2013.824709.
14. Kung HC, Jen K F, Yuan WC, et al. Influenza in China in 1977: Recurrence of influenzavirus A subtype H1N1 Bull World Health Organ 1978;56(6):913-918
15. Barberis I, Myles P, Ault SK, et al. History and evolution of influenza control through vaccination: From the first monovalent vaccine to universal vaccines J Prev Med Hyg 2016;doi: 10.15167/2421-4248/jpmh2016.57.3.642.
16. Shaw MW, et al. Reappearance and global spread of variants of influenza B/Victoria/2/87 lineage viruses in the 2000-2001 and 2001-2002 seasons. Virology 2002; doi: 10.1006/viro.2002.1719.
17. Public Health England. Surveillance of influenza and other respiratory viruses in the UK: Winter 2017 to 2018; 2018. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/740606/Surveillance_of_influenza_and_other_respiratory_viruses_in_the_UK_2017_to_2018.pdf.
18. Pebody R, et al. End of season influenza vaccine effectiveness in adults and children in the United Kingdom in 2017/18. Eurosurveillance; 2019. doi: 10.2807/1560-7917.ES.2019.24.31.1800488.
19. NHS England. The national flu immunisation programme 2020/21; 2020 https://www.england.nhs.uk/wp-content/uploads/2020/05/national-flu-immunisation-programme-2020-2021.pdf.
20. CDC. How Influenza (Flu) Vaccines Are Made; 2019. https://www.cdc.gov/flu/prevent/how-fluvaccine-made.htm.
21. Coffman RL, Sher A, Seder RA. Vaccine adjuvants: Putting innate immunity to work. Immunity; 2010. doi: 10.1016/j.immuni.2010.10.002.
22. Nanishi E, Dowling DJ, Levy O. Toward precision adjuvants: Optimizing science and safety. Curr Opin Pediatr; 2020. doi: 10.1097/MOP.0000000000000868.
23. Di Pasquale A, Preiss S, Da Silva FT, Garçon N. Vaccine adjuvants: From 1920 to 2015 and beyond. Vaccines 2015; doi: 10.3390/vaccines3020320.
24. NHS England. The national flu immunisation programme 2018/19; 2018. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/694779/Annual_national_flu_programme_2018-2019.pdf.
25. Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006;24(8):1159–69.
26. Sasaki S, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest 2011;121(8):3109–3119.
27. DiazGranados CA, Dunning AJ, Jordanov E, et al. High-dose trivalent influenza vaccine compared to standard dose vaccine in elderly adults: Safety, immunogenicity and relative efficacy during the 2009-2010 season. Vaccine 2013;31(6):861–6
28. DiazGranados CA, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014;371;635-645
29. Lee JKH, Lam GKL, Shin T, et al. Efficacy and effectiveness of high-dose versus standard dose influenza vaccination for older adults: a systematic review and meta-analysis. Expert Review of Vaccines 2018;17(5):435–443.
30. Lee JKH, Lam GKL, Shin T, et al. Efficacy and effectiveness of high-dose influenza vaccine in older adults by circulating strain and antigenic match: an updated systematic review and meta-analysis; 2020 (Submitted for publication).
31. Turner PJ, Southern J, Andrews NJ, et al. Safety of live attenuated influenza vaccine in young people with egg allergy: Multicentre prospective cohort study. BMJ 2015; 351:h6291.
32. Mohn KG, Smith I, Sjursen H, Cox RJ. Immune responses after live attenuated influenza vaccination. Hum Vaccin Immunotherap 2018;14(3):571-578
33. Belshe RB, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. New England Journal of Medicine. 2007 Feb 15;356(7):685-96.
34. Hoft DF, et al. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J Infect Dis 2011;204(6):845–853.
35. Hoft DF, et al. Comparisons of the humoral and cellular immune responses induced by live attenuated influenza vaccine and inactivated influenza vaccine in adults. Clinical and Vaccine Immunology 2017; 24(1).
36. NHS England. The flu immunisation programme 2013/14; 2013. https://www.gov.uk/government/publications/flu-immunisation-programme-2013-to-2014.
37. Rajaram S, Boikos C, Gelone DK, Gandhi A. Influenza vaccines: the potential benefits of cell-culture isolation and manufacturing. Therap Adv Vaccin Immunotherap 2020; doi: 10.1177/2515135520908121.
38. Wu NC, Zost SJ, Thompson AJ, et al. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathogens 2017;13(10): e1006682. doi: 10.1371/journal.ppat.1006682.
39. Gouma S, Zost SJ, Parkhouse K, et al. Comparison of Human H3N2 Antibody Responses Elicited by Egg-Based, Cell-Based, and Recombinant Protein–Based Influenza Vaccines During the 2017–2018 Season. Clin Infect Dis 2020;71(6):1447-53
40. Boikos C, Sylvester GC, Sampalis JS, Mansi JA. Relative Effectiveness of the Cell-Cultured Quadrivalent Influenza Vaccine Compared to Standard, Egg-derived Quadrivalent Influenza Vaccines in Preventing Influenza-like Illness in 2017–2018. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa371.
41. DeMarcus L, Shoubaki L, Federinko S. Comparing influenza vaccine effectiveness between cell-derived and egg-derived vaccines, 2017–2018 influenza season. Vaccine 2019;37(30):4015-21
42. Rubio AP, Eiros JM. Cell culture-derived flu vaccine: Present and future. Hum Vaccin Immunotherap 2018; doi: 10.1080/21645515.2018.1460297.
43. Takada K, et al. A humanized MDCK cell line for the efficient isolation and propagation of human influenza viruses. Nature Microbiol 2019; doi: 10.1038/s41564-019-0433-6.
44. CDC. Cell-Based Flu Vaccines; 2019. https://www.cdc.gov/flu/prevent/cell-based.htm.
45. Dunkle L, Izikson R, Patriarca P, et al, for PSC12 Study Team. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. New Engl J Med 2017;376: 2427–36
46. Dunkle L, Izikson R, Patriarca P, et al. Randomized comparison of immunogenicity and safety of quadrivalent recombinant versus inactivated influenza vaccine in healthy adults 18-49 years of age. J Infect Dis 2017;216(10):1219-26 doi: 10.1093/infdis/jix478.
47. Dunkle LM, Izikson R. Recombinant hemagglutinin influenza vaccine provides broader spectrum protection. Exp Rev Vaccines 2016;15(8):957-66. doi: 10.1080/14760584.2016.1203261.
Related articles
View all Articles