Flu immunisation — why all the fuss?
Mandy Galloway
Mandy Galloway
Medical writer
As practices gear up for the big push to vaccinate patients against flu, Practice Nurse looks at some of the facts and figures behind the seasonal campaign
Flu is an acute viral infection of the respiratory tract. It is highly infectious and can quickly spread, especially in closed communities. Even people with mild or no symptoms can infect others. It is easily transmitted by large droplets, or small-particle aerosols of the respiratory secretions of infected person, or by hand-to-mouth or -eye contamination from an infected surface.
Common symptoms include:
- Sudden onset of fever, chills, headache, muscle and joint pain and extreme fatigue
- Dry cough, sore throat and stuffy nose
- In young children, gastrointestinal symptoms such as vomiting and diarrhoea
The risk of serious illness from flu is higher among children under six months of age, older people and those with underlying health conditions such as respiratory disease, cardiac disease or immunosuppression, and among pregnant women. These groups are at greater risk of complications from flu such as bronchitis or pneumonia.
Common complications include bronchitis, otitis media in children, sinusitis and secondary bacterial pneumonia. Less commonly, flu can lead to meningitis, encephalitis, meningoencephalitis or primary influenza pneumonia.
Most cases in the UK occur during an 8-10 week period during the winter.
The impact of flu on the population varies from year to year and is influenced by changes in the virus that, in turn, influence the proportion of the population that may be susceptible to infection and the severity of the illness. Moderate levels of flu activity were seen in the UK in the 2014-15 flu season, with influenza A(H3N2) the predominant virus circulating for the majority of the season, with influenza B circulating towards the end of the season.
The health impact was predominantly seen in the elderly, with numerous outbreaks in care homes and levels of excess mortality significantly higher than the last notable significant H3N2 season of 2008 to 2009. Admissions to hospital and intensive care were observed, with peak ICU/HDU numbers higher than seen in the previous few seasons, but lower than the recent notable season of 2010 to 2011, which affected mainly younger adults.
Children are the main source of transmission in the population, and extending the flu programme to include healthy children should therefore reduce the spread of infection from children to other children, to adults and to those in clinical risk groups of any age.
Annual administration of flu vaccine to children is expected to substantially reduce flu-related illness, GP consultations, hospital admissions and deaths. It has been estimated that if just 30% of children had the flu vaccine, there could be 2,000 fewer deaths and 11,000 fewer hospitalisations due to flu each year.
In an average influenza season it is estimated that between 0.3% and 9.8% of 0 – 14 year old children present to a GP with flu, with incidence rates markedly higher in the younger age groups. In children, flu associated hospitalisation rates are estimated to be:
- 83–1,038/100,000 children 0-59 months old (highest in
- 16–210/100,000 children 5-17 years
In the 2015-16 flu season, all children aged 2, 3 and 4 years old on 31 August 2015 (i.e. date of birth on or after 1 September 2010 and on or before 31 August 2013) will be offered vaccination through general practice. All children of school years 1 (5-6yrs) and 2 (6-7yrs) age will be offered vaccine through locally commissioned arrangements. Primary school-aged children in areas that participated in primary school pilots in 2014/15 will also be offered flu vaccine.
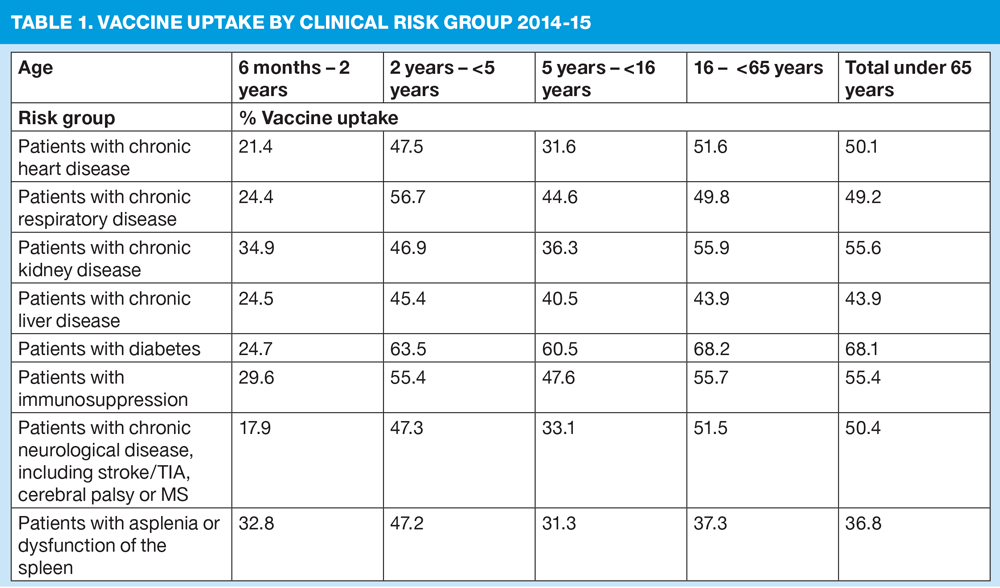
- Children aged between 6 months and 2 years, who are in a clinical risk group should also be immunised. Vaccine uptake is particularly low in the younger age groups with clinical conditions that put them at most risk of complications from flu (Table 1)
- GPs and practice staff managing the flu programme should make sure that all at-risk children have the opportunity to receive flu vaccine.
Over all age groups, increasing flu vaccine uptake in clinical risk groups is important because of the increased risk of death and serious illness if people in these groups catch flu. For a number of years only around half of patients aged 6 months to under 65 in clinical risk groups have been vaccinated. Despite those with liver disease and chronic neurological disease having some of the highest mortality rates, they have the lowest flu vaccine uptake rate amongst those in clinical risk groups. (Table 1)
Public Health England says vaccine uptake for all those in clinical risk groups needs to improve, but particularly in those with chronic liver and neurological disease, and people with learning disabilities.
THE VIRUS
There are three types of influenza viruses:
A viruses
- Cause outbreaks most years and are the usual cause of epidemics
- Animal reservoir – wildfowl, also carried by other mammals (e.g. pigs)
B viruses
- Tend to cause less severe disease and smaller outbreaks
- Burden of disease mostly in children
- Predominantly found in humans
C viruses
- Minor respiratory illness only
Changes in the surface antigens result in the flu virus constantly changing. Antigenic drift results in minor changes (natural mutations) in the genes of flu viruses that occur gradually over time. Antigenic shift occurs when two or more different strains combine. This abrupt major change results in a new subtype. Immunity from previous flu infections/vaccinations may not protect against the new subtype, potentially leading to a widespread epidemic or pandemic.
Because of the changing nature of flu viruses, the World Health Organization (WHO) monitors their epidemiology throughout the world. Each year WHO makes recommendations about the strains of influenza A and B that are predicted to be circulating in the forthcoming winter. These strains are then included in the flu vaccine developed each year.
THE VACCINE
Two main types of vaccine are available:
- Inactivated – administered by injection
- Live – given by nasal application
- None of the flu vaccines can cause clinical flu in those that can be vaccinated
Trivalent vaccines contain two subtypes of Influenza A and one type B virus (most inactivated vaccines are trivalent)
Quadrivalent vaccines contain two subtypes of Influenza A and both B virus types.
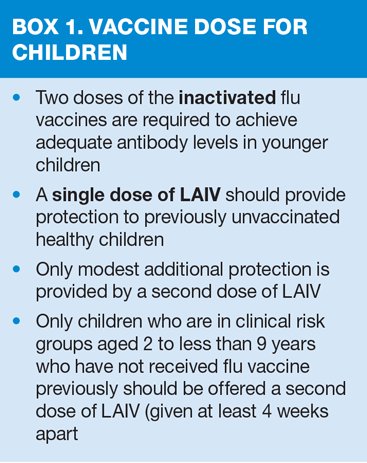
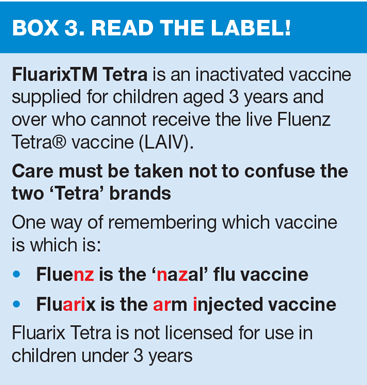
As quadrivalent vaccines may be better matched and therefore may provide better protection against the circulating B strain(s) than trivalent flu vaccines, the live attenuated intranasal vaccine (LAIV) offered to children aged 2 years and over is a quadrivalent vaccine, as is the inactivated vaccine recommended for children aged 3 years and above who cannot received live vaccine.
CONTRAINDICATIONS
There are very few individuals who cannot receive any flu vaccine:
- Confirmed anaphylactic reaction to a previous dose of flu vaccine
- Confirmed anaphylactic reaction to any component of the vaccine including gentamicin and gelatin
- Clinically severely immunodeficient due to conditions or immunosuppressive therapy
- Receiving salicylate therapy
Children with an egg allergy can be safely vaccinated with Fluenz Tetra® in any setting (including primary care and schools). Adults with egg allergy can be immunised using either an ovalbumin-free vaccine (e.g. Optaflu®, licensed from 18 years) or an inactivated flu vaccine with a very low ovalbumin content (less than 0.12 μg/ml).
Live flu vaccine not recommended for children and adolescents with severe asthma or active wheezing, e.g. those who are currently taking or have been prescribed oral steroids for respiratory disease in the last 14 days.
Children currently taking a high dose inhaled steroid – budesonide >800 mcg/day or equivalent (e.g. Fluticasone > 500 mcgs/day) should only be given live flu vaccine on the advice of their specialist.
Vaccination with Fluenz Tetra® should be deferred in children with a history of active wheezing in the past 72 hours or those who have increased use of bronchodilators in the previous 72 hours.
Where there is doubt, expert advice should be sought promptly so that the period the child is left unvaccinated is minimised. Where live flu vaccine cannot be given, it is likely that inactivated vaccine could be given instead.
SIDE EFFECTS
You can’t get flu from flu vaccine. There is a small risk that the healthcare professional administering the LAIV may be exposed to flu, but few cases have been reported. The most common adverse reactions are:
Following inactivated flu vaccine:
- Pain, swelling or redness at the injection site, low grade fever, malaise, shivering, fatigue, headache, myalgia and arthralgia
- A small painless nodule (induration) may also form at the injection site
- These symptoms usually disappear within one to two days without treatment
Following live attenuated flu vaccine:
- Nasal congestion/rhinorrhoea, reduced appetite, weakness and headache
Rarely, after live or inactivated vaccine, immediate reactions such as urticaria, angio-oedema, bronchospasm and anaphylaxis can occur.
Sources:
Public Health England. National flu programme training slide set for healthcare professionals. https://www.gov.uk/government/publications/national-flu-programme-training-slide-set-for-healthcare-professionals
Related articles
View all Articles