Vaccine Update - Summer 2016
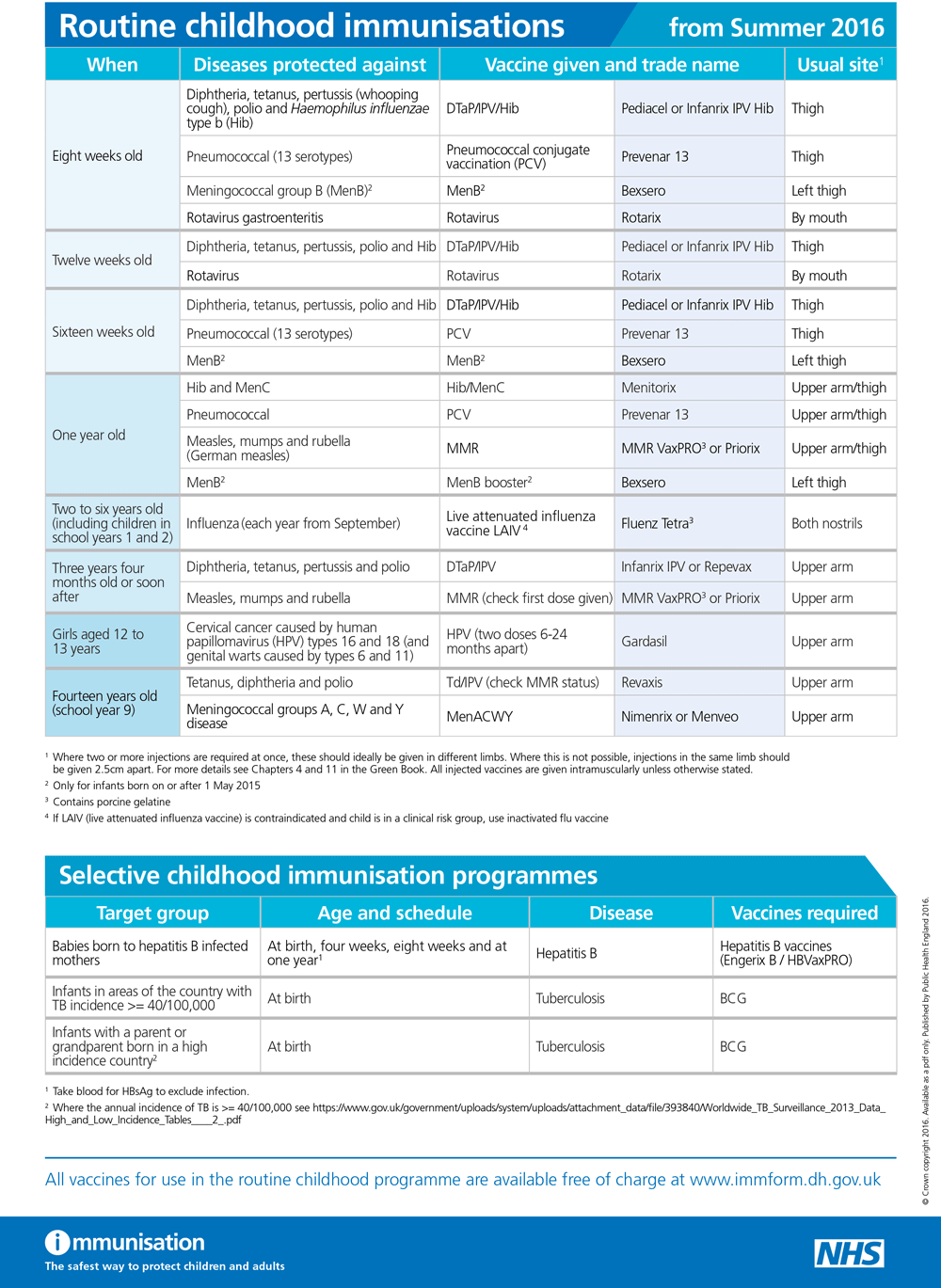
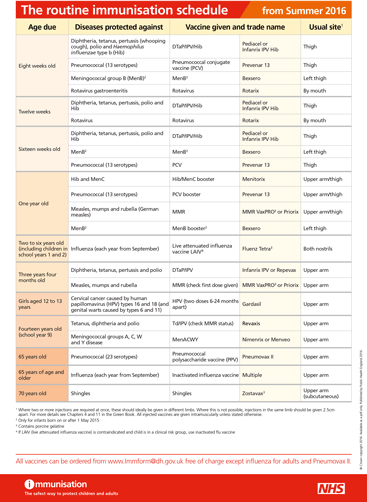
The Routine Childhood Immunisation schedule has been updated to take account of the discontinuation of the single MenC vaccination. Plus results of the first MenACWY catch-up programme; Advice on paractamol with MenB; and Measles awareness
Single MenC vaccine dropped from routine immunisation schedule
From July this year, single MenC vaccine is to be discontinued in the UK and protection against this strain of meningococcal disease will be provided by other vaccines that contain MenC (Hib-MenC or ACWY).
This change will also affect the ongoing catch-up programme for children aged 6 years or older, and young people up to the age of 25 who are unimmunised or partially immunised.
Children aged between 6 and 9 should have received at least one dose of MenC as part of the combined Hib-MenC vaccine given over the age of 1 year. Anyone who missed this dose may also require catch up for Hib, so the recommendation is to offer Hib-MenC.
Children aged 10 years or over may be eligible – or will shortly become eligible for MenACWY, so the recommendation is to offer anyone in this group who has never received a MenC-containing vaccine a single dose of ACWY vaccine.
The success of the MenC programme introduced in 1999 means there are now very few cases of invasive MenC disease, so the Joint Committee on Vaccination and Immunisation (JCVI) advised that infants no longer require vaccination against this serotype. Its inclusion in the combined vaccines is expected to maintain good herd protection, so the risk to infants will remain low.
Therefore, from next month, infants should no longer be given the dose of MenC conjugate vaccine currently given at the second primary immunisation visit at around 12 weeks.
The routine immunisations schedule has been updated to reflect changes to the MenC programme - click on the image to the right to see a larger version.
Results of first urgent catch-up for MenACWY
More than a third of eligible young people have been vaccinated against MenACWY as part of the national urgent catch-up programme, which was launched in August 2015.
The vaccine was added to the national immunisation programme last year in response to rising numbers of meningococcal W (MenW) cases. The objective was to immunise all teenagers in school years 9 – 13 before they completed academic year 13, by replacing the routine adolescent MenC booster given in years 9 or 10 with the MenACWY vaccine, with a series of catch-up campaigns targeting older teenagers.
There will be further catch-up campaigns this year and next for those currently aged 15 – 18, who will not have been offered MenACWY before. The vaccine is also being offered to older students up to age 25 who are at university as part of a ‘freshers’ programme.
The latest figures show that to date, the cumulative MenACWY coverage is 35.2%, an increase from 28.9% at the end of September 2015.
The relatively low coverage of the target group highlights the challenges of a GP-delivered vaccination programme in this age group, confirming findings from a previous HPV catch-up programme. Low coverage may be exacerbated by a significant number of the target group being away from their home address, attending university or other further education, making both invitation to the vaccination programme and monitoring more complex.
Estimates of the coverage via the school-based routine and catch-up programme will be available in late 2016 following the annual survey that takes place in September.
PHE clarifies advice on paracetamol with MenB
Public Health England has clarified its advice on administration of prophylactic liquid paracetamol when infants receive their MenB booster at 12 months.
While paracetamol is recommended for the primary vaccination, which is administered concurrently with other routine childhood vaccinations, it is not thought necessary to give paracetamol when the MenB vaccine is given on its own.
In clinical trials, the most common adverse reaction to the vaccine (Bexsero) was a high rate of fever (>38°C) in children under 2 years when it was administered at the same time as other vaccines. Prophylactic paracetamol given at around the time of the vaccination has been shown to reduce the incidence of fever requiring medical attention. In a head-to-head clinical trial of paracetamol versus ibuprofen, ibuprofen did not reduce the rate or intensity of post-vaccination fever (compared with the control group which did not receive any anti-pyretic). Therefore ibuprofen is not recommended as an alternative to paracetamol for this indication.
Some parents have expressed concern at the number of vaccines administered in a single session, but practice nurses can reassure them that there is no risk that this practice ‘overloads’ the immune system, which is able to cope with exposure to a huge number of bacteria and viruses on a daily basis from the moment the child is born.
Awareness campaign targets young people
Public Health England has launched a campaign to warn patients and healthcare professionals that anyone who has not been fully immunised is at risk of catching measles if they come into contact with the disease.
With the campaign message that measles is ‘not just a kid’s problem’, PHE has produced posters and leaflets to display in GP practices.
The leaflet warns that people with symptoms of measles should call ahead before attending their GP surgery to minimise the risk of transmission to babies who are too young to have had the MMR vaccine, or people who are immunocompromised, who may be at risk of serious complications if they are exposed to the virus.
The campaign also highlights the message that ‘It’s never to late to have your MMR vaccination’ with a timely reminder that practice nurses and other healthcare professionals who come into contact with young babies or other at risk individuals should check their own immunisation status to ensure they have had complete courses of the vaccination.
The campaign has been prompted by a recent rise in cases of measles infection. In England, 67 new measles infections were confirmed in the first three months of 2016, compared with 92 in the whole of 2015.
Most cases (82%, 55/67) occurred in two regions, London and East of England, with all but three of these linked to an outbreak imported from Italy. Scotland has also reported two confirmed measles cases in the first quarter of 2016, but none were reported in Northern Ireland or Wales.
Only one of these cases was in an individual who had received at least one dose of measles-containing vaccine. The infections were not confined to children – 40% were in adults aged 19-50 years.
Numbers of laboratory confirmed mumps remain similar to previous years, at 122 cases recorded between January and March 2016. The majority of cases (54%) were in young adults between 18 and 35 years. Two-thirds of cases were in individuals who had not received MMR vaccination in childhood.
Related articles
View all Articles