Immunisation and vaccination update
Stephanie Garner
Stephanie Garner
RGN, BSc Nurse Practitioner,
MSt (Cantab) Primary and Community Care, Independent/Supplementary Prescriber,
Association of Nurse Prescribers committee member, Advanced Nurse Practitioner — The North Brink Practice, Wisbech
According to the World Health Organization (WHO), vaccination is one of the most effective medical interventions in the world. Children are routinely immunised against major diseases, and vaccination has become a major element of worldwide public health efforts. With changes to the UK schedule imminent, Practice Nurse provides a refresher on vaccine handling and administration
Immunisation is the process of protecting individuals from infection through passive or active immunity.1 Active immunity is acquired when individuals develop antibodies after being injected with an inactive vaccine such as tetanus toxoid, or an attenuated live vaccine such as MMR. Passive immunity is provided when antibodies are passed from one individual to another such as to a foetus via the placenta or a baby via breast milk. Thus, some immunisations may be given in pregnancy to enhance passive immunity, when there is an increase in prevalence of diseases such as pertussis.
Surveillance processes are an essential element of the immunisation and vaccination programme. They are used initially to determine the burden of diseases and to establish a vaccination programme. Following vaccination implementation, surveillance is used to monitor safety and effectiveness of the programme together with continued monitoring of disease prevalence to make appropriate amendments to the schedule as necessary. This may include adding new vaccines to the programme, amending the age of administration, amending the need for, or interval between booster doses, and making adaptations such as pertussis immunisation for pregnant women.
The NMC Code2 states that, as a registered nurse, you should 'keep your skills and knowledge up to date' and 'use the best available evidence.' However, until 2005, there were no standardised guidelines to support education programmes for immunisation and vaccination training. This led to inequalities within the training being offered. In 2005, the Health Protection Agency published two documents, The National Minimum Standards for Immunisation Training3 and The Core Curriculum for Immunisation Training.4 These guidelines detail essential core topics that should be included and recommend frequency and duration of training programmes together with guidance on obtaining and maintaining competency.
STORAGE AND HANDLING OF VACCINES
Vaccines are delicate biological substances with pre-determined shelf lives. For them to work effectively they are dependent upon correct storage and transportation procedures.
The cold chain
The cold chain is a system for transporting and storing vaccines within safe temperature ranges from the time of manufacture, through any transportation and storage until its administration to a patient. Most vaccines need to be stored at 2ºC - 8ºC but it is essential that individual manufacturer recommendations are followed.
Maintaining the cold chain is essential:
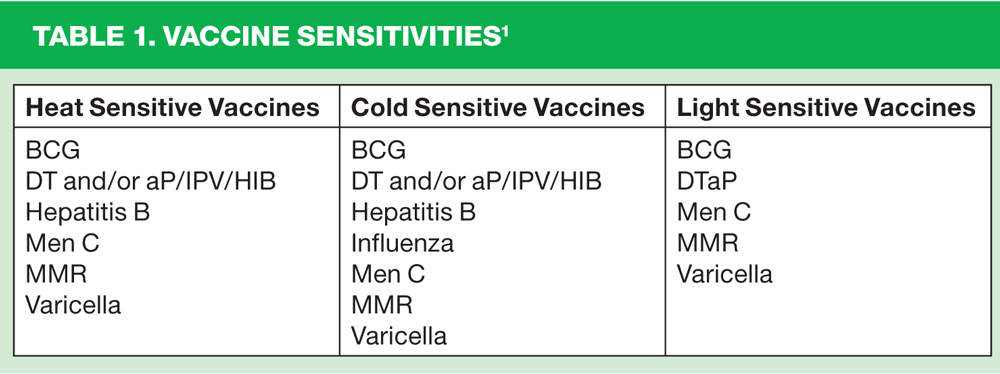
- To ensure efficacy and potency of the vaccine, damage can be caused by extremes of temperature and external factors (see Table 1)
- To comply with the manufacturer's recommendations
- To have confidence in the product being effective and providing the maximum benefit and least risk to the patient.
Effects of temperature and external factors
Repeated heat exposure has a cumulative effect and is irreversible; it increases the risks of vaccine reactions after administration.
If a vaccine is exposed to freezing it loses its potency and will therefore no longer be effective. Most vaccines will be destroyed at 0ºC. Freezing may also damage glass vials, which may allow bacterial contamination or result in minute glass shards being present in the vaccine.
Direct sunlight or fluorescent light can also damage some vaccines and reduce their potency.
When vaccines are delivered a designated person should be responsible for ensuring the cold chain has been maintained throughout its transport, that the vaccines are not damaged and that stock is rotated. Overstocking should be avoided as vaccines need free air circulating around them: any vaccines pushed to the sides or back of the fridge can inadvertently freeze and the cold chain will be broken.
Vaccines should be stored in a dedicated vaccine fridge as recommended by the MHRA. Pharmaceutical fridges have a fan that ensures a uniform temperature throughout, they are fitted with maximum and minimum thermometers that can be read externally thus preventing unnecessary door openings, are lockable and often fitted with alarms to warn when temperature levels are outside the recommended 5ºC — 8ºC.
Vaccines should still remain in the cold chain if they are to be taken to a satellite clinic or to be administered in the home. The gold standard is for vaccines to be transported in a vaccine specific cool box that has an integral thermometer.
VACCINE ADMINISTRATION
In primary care, vaccines are often administered under a patient group direction (PGD). Before administering any immunisation under a PGD you should have received training in administering from a PGD, and have undertaken training in administering immunisations and vaccinations as detailed above. In addition to this, up to date resuscitation and anaphylaxis training is essential. Vaccines should not be given if there are no facilities to provide basic life support and/or anaphylaxis management.
When administering vaccines current best practice guidelines should be followed, local PGDs, guidelines and protocols should reflect advice from the Joint Committee on Vaccination and Immunisation (JCVI), the Department of Health Green Book,5 letters and updates from the Chief Medical Officer (CMO) and NHS vaccine updates (see useful resources).
Skin cleaning
While studies have demonstrated that skin cleaning with isopropyl alcohol reduces the bacterial count on the skin,6 there is no substantive evidence to support any increase in infection rates when not cleaning the skin. The use of alcohol swabs can in fact damage live vaccines such as MMR if the alcohol is not allowed to dry fully for a minimum of 30 seconds before an injection is given.7 It is widely considered best practice not to use alcohol swabs. Should the area be dirty, soap and water are recommended to remove any transient bacteria that may be present.
Needle size
Needles should be the appropriate size to deliver the vaccine to the correct site in the individual patient. An injection being delivered intramuscularly (IM) will require a longer needle than one for a subcutaneous (SC) or intradermal (ID) injection. While it may appear tempting to use the smallest needle possible in infants, by using the longer 25mm long for IM injections in infants it ensures the vaccine is delivered into the muscle and reduces the risk of local reactions.8
The gauge of the needle should also be considered: the wider the bore, the larger the area the vaccine can disperse over and the vaccine is administered at a much lower pressure, thus again reducing the incidence of localised reactions.
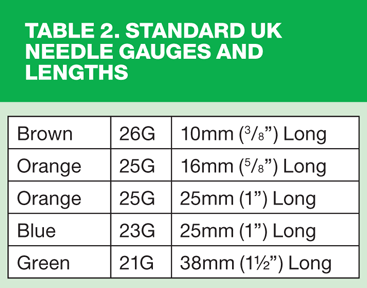
23 Gauge (blue) or 25 Gauge (orange) should be used for infants and a 23 Gauge (blue) used for adults.1 An intradermal injection should only be given with a 26 Gauge (brown) needle. Standard needle sizes are shown in Table 2.
Green book recommendations
The DH recommends using a 25mm needle with either a 23 Gauge (blue) or 25 Gauge (orange) for intramuscular injections for infants, children and adults and suggests that the smaller 16mm orange needle should be reserved only for pre-term or very small infants. It may be also be necessary to use a 38mm (green) needle for some larger adults.
VACCINE PREPARATION
Attention to detail is essential. You should ensure you have the correct vaccine, that it is in date, has been stored correctly and is not damaged in any way.
Many vaccines are now available ready prepared in prefilled syringes, however freeze dried (lyophilised) vaccines will need to be reconstituted before use. Adherence to good practice guidance prevents cross contamination and the risk of adverse reactions.
1. Ensure good hygiene practices are followed throughout
2. Check expiry dates of both the vaccine and the diluents
3. Using only the supplied diluent, draw up the diluent with a 21G (green) needle and add slowly to the vaccine
4. Once the reconstituted vaccine is ready for use, change the needle to one appropriate for your patient
5. Only mix vaccines if there is a license and it is recommended to do so
- If a multi dose vial is used ensure you follow the manufacturer's recommendations for shelf life and ensure accuracy when measuring the dose to be administered.
PATIENT PREPARATION
Informed consent should be gained from the patient and/or carer before every vaccination following a discussion of both the risks, including potential side effects and benefits.9 Consent may be written, verbal or implied and should be recorded in the patient's record.
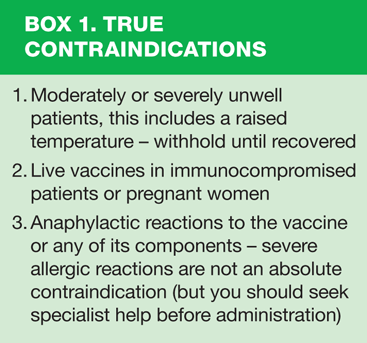
Prior to administration you should ensure that the vaccine is appropriate for your patient. Checking against patient records including personal child health records is important to avoid duplication, and the immunisation schedule should be checked to confirm the vaccination is required. Does the patient have any contra-indications or previous reactions to the vaccine? See box 1.
Positioning the patient
To ensure safe and effective administration it is important to have the patient positioned correctly: older children or adults may sit, or if prone to fainting or are anxious, then they may lie down. The appropriate limb should be fully exposed and the patient encouraged to relax the muscle either by hanging the arm by their side or placing their hand on their hip.
Infants or young children should be seated on their parent or carer's lap with the exposed limb securely held.
INJECTION TECHNIQUE
Site of injection
Sites should be chosen to avoid major vessels and nerves. The DH5 recommends that for infants under one year old the anterolateral aspect of the thigh should be used; WHO, however, recommends this site should be used until 15 months of age.
For children and adults the deltoid muscle should be used or if preferred the anterolateral aspect of the thigh.
The buttock is no longer recommended as a site for injection due to the increased risk of injecting into fat or causing damage to the sciatic nerve.
If more than one injection is to be given at the same time use the same site in different limbs. If more than two are to be given, you can use the same limb as long are the sites are at least 2.5cm apart. Carefully document which site was used for each vaccine.
There are three techniques for administering immunisations and vaccinations
- Intramuscular (IM)
- Subcutaneous (SC)
- Intradermal (ID)
Intramuscular injections
The IM technique is used for the majority of immunisations and vaccinations. The skin should be stretched flat with one hand and the needle inserted at 90o to the skin (this is also known as the Z-Track method). There is no need to aspirate, and only gentle pressure should be applied after administration.
Subcutaneous injections
The deep SC injection technique is usually reserved for patients with bleeding disorders. For a SC injection the skin should be bunched up and the needle inserted at a 45o angle to the skin. Again, there is no need to aspirate, and gentle pressure should be administered if required.
Intradermal injections
The intradermal route is used for administration of BCG vaccine. This is a specialist skill that requires specific training and assessment of competency. The skin is stretched and with the needle bevel upwards the needle should be inserted 2mm into the superficial dermis layers. As the injection is administered resistance should be felt and a raised bleb should appear.
FOLLOWING ADMINISTRATION
All equipment should be disposed of following local protocols and guidelines. Be careful to observe the recommended timing that the vaccine has been out of the cold chain, and when it should be discarded.
Records should be completed detailing the vaccine or vaccines given, the site(s) of injection, batch numbers, expiry dates and the name of the person administering the vaccine. Information should be recorded in the patient medical record and if applicable in the personal child health record (Red book) and child health information system (CHIS).
Any post immunisation information should be given to the patient or carer with advice on action to take should any adverse reactions occur.
CONCLUSION
Immunisation and vaccination is second only to clean water as the most important programme in preventing infectious diseases. To ensure vaccines are administered safely and effectively it is essential that all vaccine givers work within the NMC code2 by having regular training that meets the national standards and are aware of evidence based, reliable sources to ensure they are practicing using the most up to date guidelines and recommendations.
REFERENCES
1. Health Protection Agency (2013) Available at: www.hpa.org.uk
2. Nursing & Midwifery Council (2008) The code: Standards of conduct, performance and ethics for nurses and midwives. Nursing & Midwifery Council. London
3. Health Protection Agency. The National Minimum Standards for Immunisation Training. Health Protection Agency. London;2005
4. Health Protection Agency The Core Curriculum for Immunisation Training. Health Protection Agency. London;2005
5. Department of Health (2013) The Green Book. Available at: https://www.wp.dh.gov.uk/immunisation/files/2012/09/Green-Book-updated-180113.pdf
6. Del Mar CB, Glasziou PP, Spinks AB, et al. Is isopropyl alcohol swabbing before injection really necessary? Medical Journal of Australia 2001:74: 306
7. Department of Health (1996). The Green Book. Available at: https://www.wp.dh.gov.uk/immunisation/files/2012/07/Green-Book-original-2006.pdf
8. Diggle L, Deeks J. Effect of needle length on incidence of local reactions to routine immunisation in infants aged four months: randomised controlled trial. BMJ 2000; 321:931-3
9. Department of Health (2001) Seeking consent: working with children. Available at: www.dh.gov.uk/assetRoot/04/06/72/04/04067204.pdf
10. Diggle L. (2007) Injection technique for immunisation. Practice Nurse 2007; 33(1)
Related articles
View all Articles