Why GLP-1 should not be relegated to the 4th division in type 2 diabetes
Beverley Bostock-Cox
Beverley Bostock-Cox
RGN, MSc, QN
Nurse Practitioner Mann Cottage Surgery
Education Lead Education for Health Warwick
When should you consider a GLP-1 for patients with type 2 diabetes, how do you select the right one for the individual, and what does the NICE guideline say?
In 2015, NICE published updated guidance on the management of type 2 diabetes (T2D).1 The section on pharmacological interventions recommended metformin first line, unless contraindicated, in line with international guidance from America and Europe.2
Once intensification of therapies is required, NICE states that the decision regarding which class of drug to use next should be tailored to each individual. The ADA/EASD guidelines go further by saying that these decisions should be based on the patient’s lifestyle, age, length of time of having diabetes, weight, renal and cardiac considerations and the risk:benefit profile for each option. As a result, second line drugs might include dipeptidyl peptidase 4 (DPP-4) inhibitors (-gliptins), SGLT2 inhibitors (-flozins), pioglitazone or even a sulfonylurea (SU). However, one class of drug, the glucagon-like peptide-1 (GLP-1) mimetics, which had previously been recognised as having a role at the first intensification of therapy (i.e. as a second line drug after metformin) was, in the 2015 NICE guidelines, only suggested as an option if triple therapy was not working.
Many people with experience of treating diabetes were surprised about this as the GLP-1 therapies have some unique advantages for people with T2D. In this article, we will consider the role of GLP-1 therapies in the management of T2D and challenge NICE’s view that they should be relegated to the 4th division.
WHAT ARE GLP-1s?
GLP-1 mimetics (or agonists) copy the action of naturally occurring GLP-1, which is a hormone that is released from the gut via the incretin system in response to food intake.3 As a result of GLP-1 being released, insulin is secreted. GLP-1 also suppresses glucagon secretion and delays gastric emptying. GLP-1 secretion is thought to be deficient or even absent in some people with type 2 diabetes. The GLP-1 therapies, then, target the incretin system and act in the same way as naturally occurring GLP-1 to improve glycaemic control and aid weight loss. GLP-1 mimetics are injectable therapies that are usually used as an add-on treatment for patients whose blood glucose is inadequately controlled with their current regimen of oral hypoglycaemic agents (OHA).2 Some GLP-1 therapies can be used alone, however (see table below). As well as reducing blood glucose through the action described above, their ability to reduce appetite and assist weight loss also improves glycaemic control.4 GLP-1s rely on the presence of glucose in the gastrointestinal (GI) tract to work meaning that hypoglycaemia is very unlikely to occur.
WHAT ADVANTAGES DO THEY OFFER?
GLP-1 therapies improve glycaemic control while offering the additional benefit of increasing feelings of satiety (fullness) and reducing appetite and are therefore linked to potential weight loss.5 As a result of their dependence on the presence of glucose in the GI tract to work, GLP-1 therapies have been shown to carry a very low risk of hypoglycaemia and it is often only the combination of a GLP-1 with an SU which increases this risk. There is also evidence that they can reduce systolic blood pressure,6 which is an advantage in T2D when many patients have hypertension requiring treatment with two or more different antihypertensive agents.2
WHAT ARE THE DISADVANTAGES OF GLP-1 THERAPY?
The side effects of GLP-1 drugs are usually transient and mild, and include nausea and dyspepsia resulting from the reduced gastric emptying (increased transit time of food through the gut). Although there have been concerns about a possible increased risk of pancreatitis and carcinoma of the pancreas in incretin therapy users, both the USA Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have stated that evidence shows no statistically increased rates of these conditions in people taking DPP-4 inhibitors or GLP-1 therapies.7 As a result, clinicians may want to explain to people that this risk has been highlighted but that evidence is reassuring and that the risk:benefit ratio is likely to be positive for the vast majority of people. Caution should be used in people with a previous history of pancreatitis, however.
HOW SHOULD THEY BE USED?
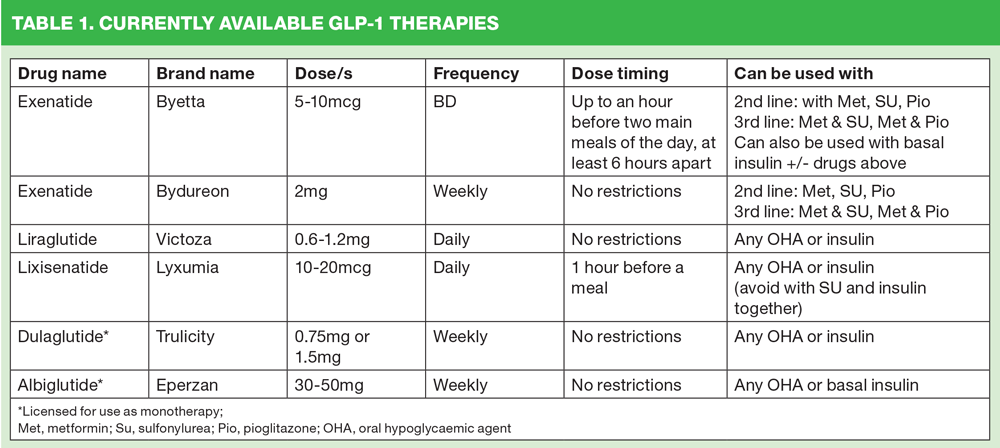
Although NICE guidance suggests GLP-1 therapies should be initiated after triple therapy has failed, they are actually licensed for use at every stage of the diabetes medication algorithm. Dulaglutide and albiglutide both have licences as monotherapy and the others can be used at first and second intensification. Most are licensed to use with insulin (see Table 1 for details). Furthermore there is evidence that GLP-1 therapies may be beta cell-sparing, allowing the pancreas to recuperate and extending the lifespan of these insulin-producing beta cells.8 This is in contrast to traditional and commonly used therapies such as SUs which have been shown to exhaust the pancreas,9 potentially increasing the risk of pancreatic ‘failure’ leading to the need for insulin initiation. This argument supports the earlier use of GLP-1 therapies.
CHOOSING A GLP-1
There are currently six GLP-1 drugs available for prescribing. Information on each product can be found on the Electronic Medicines Compendium website: https://www.medicines.org.uk/emc/
Exenatide: Exenatide (standard release – Byetta) was the first GLP-1 to be launched in the UK. Byetta is given by a twice-daily injection but exenatide is more commonly given as a once weekly delayed release injection, known as Bydureon. Bydureon is given at a dose of 2mg and can be combined with metformin, a sulfonylurea, pioglitazone, metformin and a sulfonylurea or metformin and pioglitazone. It can be given at any time of day. Bydureon is not recommended in patients with moderate or severe renal impairment but it can be used in hepatic impairment.
Liraglutide (Victoza) is given once daily and is not meal dependent in terms of timing. It can be used with oral hypoglycaemic agents and/or basal insulin. The starting dose is 0.6mg increasing after one week to 1.2mg. It should not be used in severe renal impairment or in patients with hepatic impairment.
Lixisenatide (Lyxumia) is a once daily injection given an hour before any meal. The starting dose is 10mcg, increasing after 14 days to 20mcg. It can be added to oral hypoglycaemic agents or basal insulin. However, it should not be used with a sulfonylurea and insulin together as this increases the risk of hypoglycaemia. It can be used with caution in moderate renal impairment but should not be used in severe renal impairment. It can be used in hepatic impairment.
Dulaglutide (Trulicity) is a once weekly GLP-1 which can be used as monotherapy (0.75mg) or in combination with other oral hypoglycaemic agents and/or insulin (1.5mg). It should not be used in severe renal impairment but can be used in hepatic impairment.
Albiglutide (Eperzan) is a once weekly GLP-1 which can be used as monotherapy or in combination with other oral hypoglycaemic agents and/or basal insulin. The initial dose is 30mg although this can be increased to 50mg if required. It should not be used in severe renal impairment. No dose adjustment is required in hepatic impairment.
CHOOSING A GLP-1
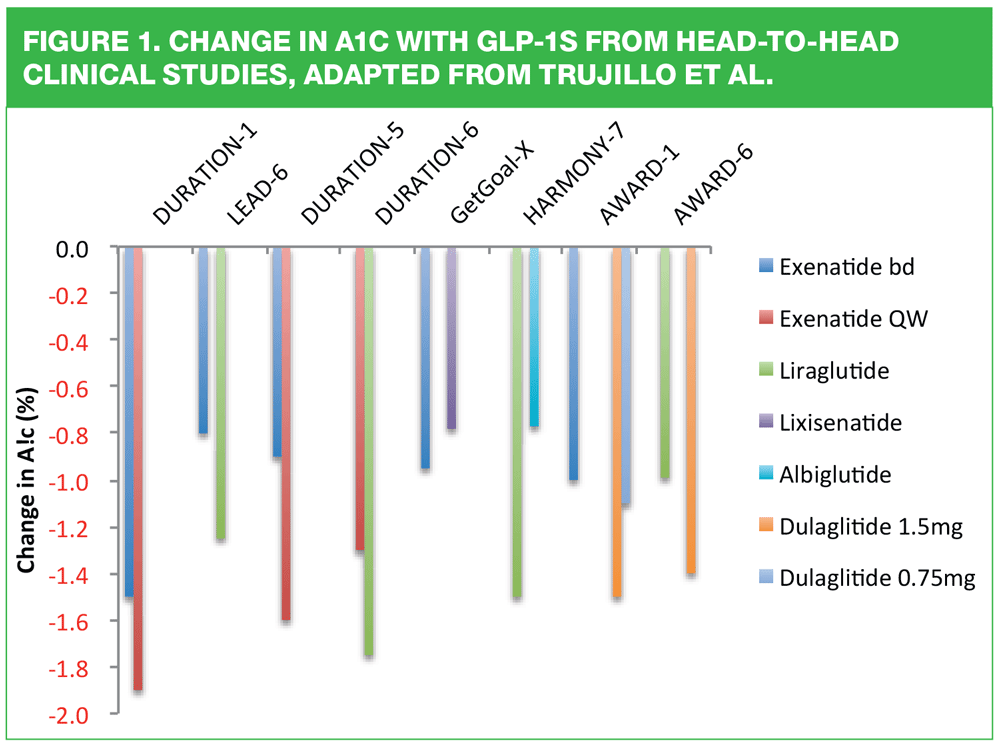
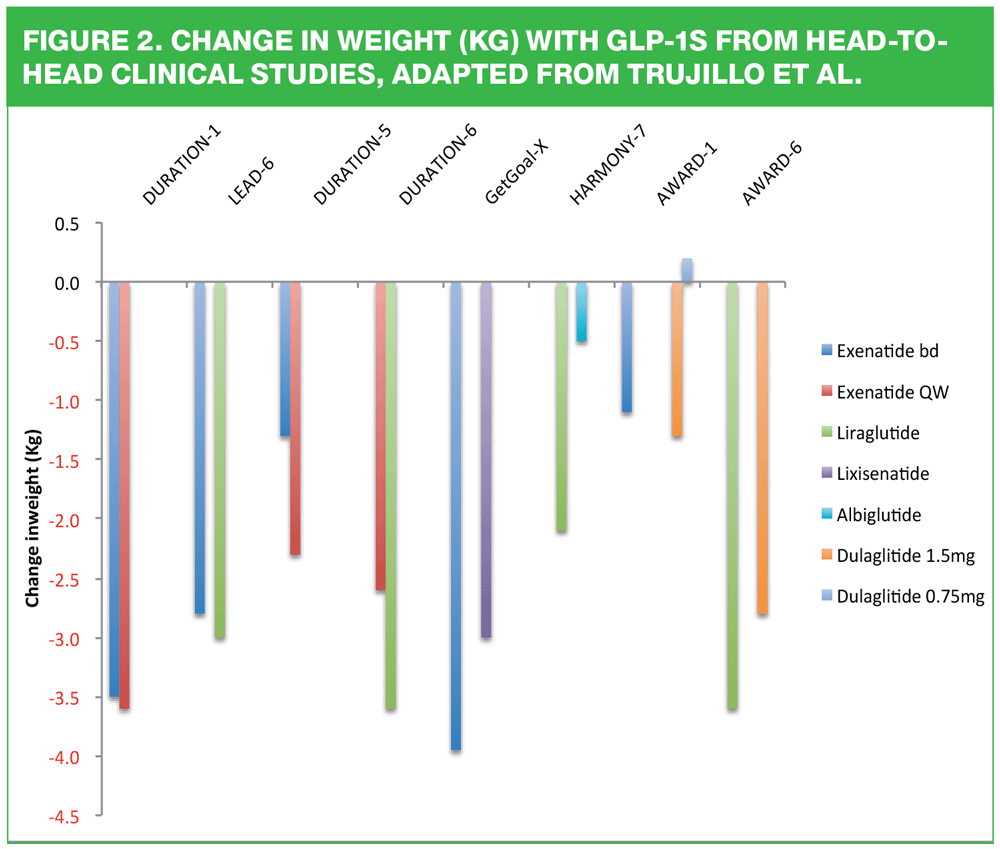
The decision about which GLP-1 to use should be a collaboration between the patient and the clinician. Consideration should be paid to the licensing restrictions noted in Table 1 in terms of other medication being taken, as well as renal and hepatic function. It is likely that patients will express a preference in terms of how frequently they would like to inject, too. However, there are differences in drug effectiveness in the class as demonstrated below. The figures from the various trials reported here indicate that Bydureon and Victoza and possibly Trulicity may have an advantage over other drugs in class for glycaemic and/or weight benefits. This is why it is important to consider the role of prescribing formularies when selecting the most appropriate drug for each patient as formularies can only be written for populations; they are not written for the person sitting in that particular consultation. Nurses should remember the NMC’s 4 Ps in order to deliver patient-centred care:
Prioritise people
Practise effectively
Preserve safety
Promote professionalism and trust
Nonetheless, it goes without saying that there are cost difference between the drugs and if all other aspects are equal (which is rarely the case, in fact) then the drug with the lowest acquisition cost should be chosen.
DEVICES
By and large the devices are similar to insulin pens and are straightforward to use. Instructions on each device are available from the manufacturers (see EMC website) and clinicians and patients should be familiar with them before use. Bydureon is slightly different in that the pen requires the drug to be pre-mixed before use by merging two compartments of ingredients into one through a simple twisting action followed by rolling the device to ensure the product is fully mixed and ready to use. Eperzan also requires pre-mixing but must then be left to stand for 15 minutes pre-injection to ensure the powder has dissolved.
GLP-1 WITH INSULIN – COMBINATION THERAPY
As noted above, some, but not all of the GLP-1s can be used with insulin. There is also one combination product which contains a GLP-1 with insulin: Xultophy. NICE has issued guidance on the use of this product.10 It is a once daily injection that combines liraglutide with insulin degludec, an ultra-long acting basal insulin, and which has been shown to reduce HbA1c by around 20mmol/mol over a year when added to OHAs.10 Using the two drugs together showed a greater benefit than using either drug alone.11 This combination therapy gives the option of combining the glycaemic benefits of insulin and the GLP-1 while reducing the risk of weight gain which is a common side effect of insulin and allowing a lower dose of insulin to be used, which will also reduce the risk of hypoglycaemia and weight gain. Xultophy is administered in dose steps. The pen device delivers 1 to 50 dose steps in each injection with a recommended starting dose of 10 dose steps and a maximum daily dose of 50 dose steps, which would equate to 50 units of insulin degludec and 1.8 mg of liraglutide.
CONSIDERATIONS FOR PRACTICE
People starting on a GLP-1 should have the treatment most suitable for them based on renal and hepatic function along with a preference for once (or twice) daily or once weekly injections. Injection technique should be taught along with information on the safe disposal of sharps and pen devices. As with any other diabetes treatments, people starting on GLP-1 therapies should be advised on possible side effects and sick day rules.
These therapies are some of the most expensive drugs used to treat diabetes and it is important to ensure that they are used in the group of people who are likely to benefit the most. NICE states that these people are those with a BMI of 35kg/m2 or more or those with a lower BMI who might have physical or psychological co-morbidities which might benefit from the weight loss, or who need to avoid insulin, perhaps for work-related issues. The NICE guidelines also recommend that the effect of the drug is monitored in terms of both glycaemic control and weight loss and that it should be discontinued if there is a less than 11mmol/mol reduction in HbA1c after 6 months of treatment or if weight has not dropped by 3% or more in the same period.1
It is worth considering that the newer class of drugs known as SGLT-2 inhibitors also improve glycaemic control while promoting weight loss and are taken orally, rather than by injection making them potentially more acceptable to most people.1
CONCLUSION
Although the NICE guidelines place GLP-1 therapies at 4th line after the failure of triple therapy, there are many reasons for considering these drugs at an earlier stage. Their cost makes it important that patient suitability is determined with care and that those patients who do not respond adequately are taken off treatment. Trials suggest that there are differences between the drugs in this class in terms of efficacy and there are also licensing difference between the different type of GLP-1 agonists. Clinicians should be mindful of these differences and should prescribe the drug most suitable for their patient. Patients should be involved in the decision-making process and should be taught how to prepare and inject the product correctly. When used appropriately, GLP-1 agonists can improve glycaemic control, reduce appetite and assist with weight loss while potentially sparing the beta cells in the pancreas.
REFERENCES
1. NICE NG28. Type 2 diabetes in adults: management, 2015 https://www.nice.org.uk/guidance/ng28
2. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in Type 2 Diabetes. Diabetes Care 2015;38:140-149. http://care.diabetesjournals.org/content/38/1/140
3. Dicembrini I, Paia L, Rotell CM. from theory to clinical practice in the use of GLP-1 receptor agonists and DPP-4 inhibitors therapy. Exp Diabetes Res 2011 doi:10.1155/2011/898913
4. Nauck MA. Incretin-based therapies for type 2 diabetes mellitus: properties, functions and clinical implication. Am J Med 2011;124(Suppl): S3-18
5. Garber AJ. Long-Acting Glucagon-Like Peptide 1 Receptor Agonists Diabetes Care 2011;34(Suppl 2): S279-S284
6. Okerson T, Yan P, Stonehouse A, et al. Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. Am J Hypertension 2010 3:334–9
7. Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs – FDA and EMA assessment. N Engl J Med 2014;370:794–797
8. Vilsbøll T. The effects of glucagon-like peptide-1 on the beta cell. Diabetes Obes Metab 2009;11(Suppl 3):11-8
9. Shin MS, Yu JH, Jung CH, et al. The duration of sulfonylurea treatment is associated with β-cell dysfunction in patients with type 2 diabetes mellitus. 2012;14(11):1033-42.
10. NICE. Type 2 diabetes: insulin degludec/liraglutide (Xultophy), 2015.
https://www.nice.org.uk/advice/esnm60/chapter/Full-evidence-summary
11. EmC. Xultophy SPC, 2016 https://www.medicines.org.uk/emc/medicine/29493
12. Trujillo J, Nuffer W, Ellis S. GLP-1 receptor agonists: a review of head-to-head studies. Ther Adv Endocrinol Metab 2015;6:19-28.
Related articles
View all Articles