The new NICE guidance for type 2 diabetes - was it worth the wait?
Beverley Bostock-Cox, RGN, MSc, QN, Nurse Practitioner, Moreton-in-Marsh, Education Lead, Education ...
Beverley Bostock-Cox, RGN, MSc, QN, Nurse Practitioner, Moreton-in-Marsh, Education Lead, Education for Health, Warwick
As reported in Practice Nurse last month, NICE has published its long awaited updated guideline on type 2 diabetes. Here we look at the guidance in detail and extract the key messages for general practice nurses
After a long wait and an embarrassing false start, the new NICE guidance for managing type 2 diabetes was finally published in December 2015.1 The publication of these guidelines had been delayed by the rejection of the prescribing advice contained in the first draft so many people were particularly interested to see how NICE would respond to this feedback and how the revised guidance would look. Some doubted that NICE would make significant changes while others thought that the guidelines would be untenable without those changes. All was revealed in the pre-Christmas launch of the latest and final version and the general response was a collective sigh of relief.
In this article we will review the key messages of the guidelines and consider anything new which could impact on practice from 2016 onwards. As well as some new recommendations, NICE underlined some of the most important performance indicators from previous guidance that were worth repeating and these will also be discussed here.
THE LINK BETWEEN NICE AND THE NMC
As discussed in Practice Nurse previously, nurses are expected to abide by their code of conduct, using reflective practice which focuses on the 4 Ps of the code –
1 – Prioritise people
2 – Practise effectively
3 – Preserve safety
4 – Promote professionalism and trust2, 3
One of the most important features of the new NICE guidance is the focus on patient-centred care in line with the 4 Ps. NICE stresses the importance of negotiated targets and personalised care plans which reflect the needs of each individual person with diabetes. For example, many people with diabetes will have other co-morbidities which may concern them as much as or even more than their diabetes and this needs to be recognised when planning care and providing support with self-management. In terms of prescribing for glycaemic control, which was the most contentious issue in the previous draft guidelines, there is now clear recognition of the importance of tailoring treatment to suit the individual and their lifestyle. All of this provides an excellent congruence between how nurses work and what the guidelines recommend – a congruence which has perhaps been less evident in previous diabetes guidelines.
EDUCATION AND SUPPORT TO SELF-CARE
NICE underlines the role of structured patient education (such as DESMOND and other similar courses) as a way of enabling people with diabetes to understand more about their condition along with the rationale for lifestyle and pharmacological interventions. A new recommendation is that the effect of this education is audited. This audit may be carried out most effectively by the team that delivers the education but from a primary care perspective, it does throw up some interesting challenges. How do we measure the effect of education on people’s understanding of the causes and management of diabetes? What happens to people who have not been referred on diagnosis but who might still benefit from attending later in the disease trajectory, especially when places are often limited to people who are newly diagnosed? How do we ensure that the education provided in practice is structured and standardised so that all members of the primary health care team are giving the same key messages? It may be that a priority for 2016 should be to look at how ongoing education is delivered in the practice and consider the resources that are available to patients. This could be a useful part of a reflective exercise for general practice nurses (GPNs) which could be used for revalidation purposes, as well as having a beneficial effect on patients and the practice.
Care planning is endorsed in the guidelines, although recent evidence suggests that only 5.4% of people with a long term condition actually have a care plan.4 NICE recommends that consideration is given to any disabilities, particularly visual problems, when care planning but it might be good to see a more proactive approach to this concept in general. Diabetes UK produces some excellent resources to help people with care planning and these can be accessed via their website (see resources below).
TARGETS
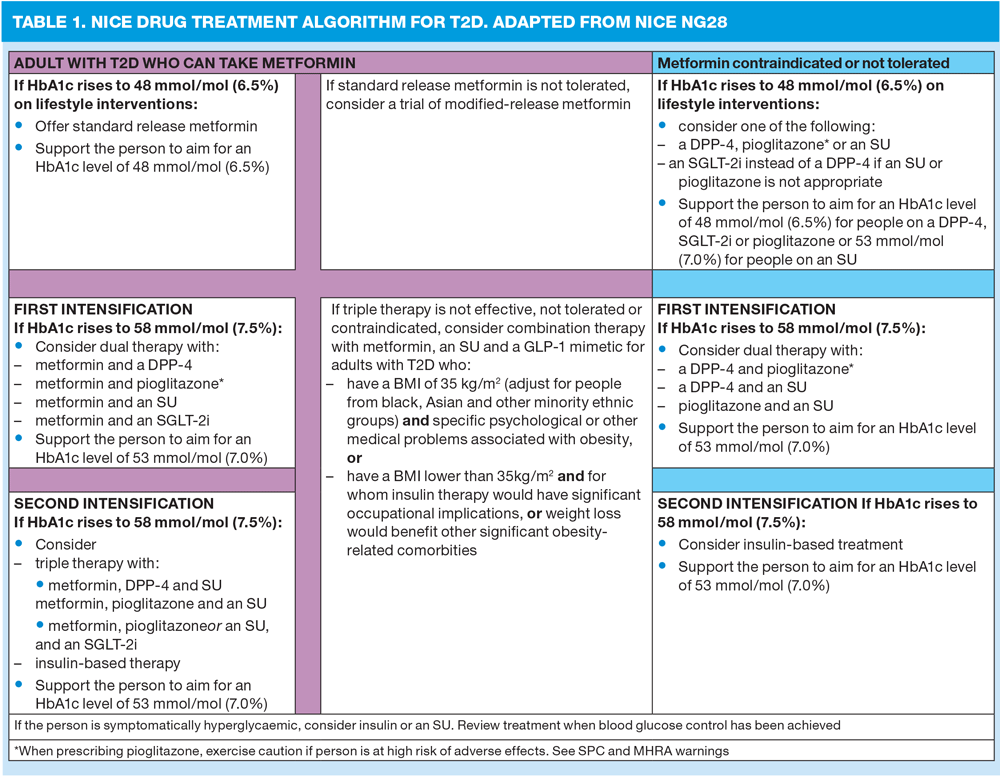
The focus on purely numerical targets has changed in recent years allowing greater leeway and recognition of the needs of individuals. NICE recommends that adults should be involved in decisions about targets (e.g. HbA1c) although the overall aim should be to reach and maintain the recommended targets (as this is likely to reduce the risk of cardiovascular complications) unless the individual suffers a negative impact on their quality of life by doing so. The ideal target for HbA1c is 48mmol/mol or less if the individual is on one drug which does not have a risk of hypoglycaemia (hypos) or 53mmol/mol or less if they are on monotherapy which does carry a risk of hypos. If a patient on monotherapy has an HbA1c of 58mmol/mol or more they should aim to look at further changes to their lifestyle and have their treatment intensified. Once good control has been established, NICE advises that HbA1c should be rechecked 6 monthly – in other words not just at the annual review. In cases where people’s HbA1c seems unusually low, NICE recommends that consideration is given to any underlying cause such as weight loss or renal impairment.
ADVICE ON STARTING ORAL HYPOGLYCAEMIC AGENTS (OHGA)
NICE reiterates its previous advice that metformin should be the first line oral hypoglycaemic agent unless contraindicated (rather than not tolerated). In cases where it is contraindicated (for example if the eGFR is less than 30ml/min) then a DPP4 inhibitor, pioglitazone or a sulfonylurea may be considered (assuming the contraindication to metformin is not also a contraindication to one of these drugs, of course). For cases where people cannot tolerate metformin, usually because of gastrointestinal side effects, NICE has retracted its advice in the draft guidance and has now endorsed the use of modified release metformin. The new guidance does remind clinicians, however, that gentle titration of metformin is likely to improve tolerability. This often means starting a patient on 1 tablet daily with a meal for the first week and then increasing to 1 tablet twice daily if the patient is not suffering any ill effects. Repaglinide is another option for people who cannot tolerate metformin, but this is rarely used as it has to be taken three times a day with meals and can also prove problematic if further intensification is needed due to licence limitations.5 Repaglinide featured highly in the draft guidance but has rightly been demoted in the final version because of these issues.
NICE recognises that sulfonylureas and insulin can both be useful ‘rescue’ therapies for people with symptomatic hyperglycaemia – those with T2D who present with polyuria and polydipsia for example.
INTENSIFICATION OF TREATMENT
NICE suggests that if metformin is not enough to control blood glucose levels, the next option would be to use a DPP4 inhibitor, pioglitazone or a sulfonylurea.
If metformin is contraindicated, suggested dual therapies are:
- DPP4 and pioglitazone
- DPP4 and sulfonylurea
- Pioglitazone and sulfonylurea
If triple therapy is required, the following interventions should be considered:
- Metformin/DPP4/sulfonylurea
- Metformin/pioglitazone/sulfonylurea
- Initiation of insulin therapy may be more appropriate at this stage for patients who cannot take metformin due to contraindications. [text] Insulin delivery guidelines written for type 1 diabetes should be used when considering insulin in T2D.6
Glucagon-like peptide-1 (GLP1) mimetics are mentioned in a ‘new for 2015’ section of the guidelines. The advice is very specific albeit not exactly ground-breaking and some might say a little disappointing. The recommendation is that if triple therapy with metformin and two other OHGAs is not adequate, not tolerated or contraindicated, combination therapy using metformin, a sulfonylurea and a GLP-1 should be considered in people who have a BMI of 35 kg/m2 or more and where this obesity is causing psychological or medical problems. This combination may also be considered in people with a lower BMI but for whom insulin therapy is not appropriate due to their occupation or where weight loss would benefit other co-morbidities. The disappointment mainly relates to the fact that GLP1s have much to offer specific individuals at an earlier stage than ‘failed triple therapy’ (improved glycaemic control, weight loss, no or very low risk of hypos) and clinicians should use their clinical judgement when making decisions about the most appropriate and cost effective use of GLP1s.
NICE is a little coy when it comes to discussing the newest class of OHGAs – the SGLT2 inhibitors. The guidelines say that treatment with combinations of drugs including SGLT2 inhibitors may be suitable for some patients, a statement which surely applies to all of the drug classes so why the need to mention this specifically in relation to SGLT2s? In reality these drugs have much to offer as a first intensification – lowered blood glucose, weight loss and no or low hypo risk.7 Indeed, the recently published EMPA-REG study has caused a stir with the hint of a promise of CVD benefits.8 NICE may be a little shy about offering a clear endorsement for this class of drugs but the early indications are that these are a very useful adjunct to other therapies.
Another key reminder in the new guidelines is that treatment that does not work should be stopped. Specific targets are mentioned in relation to the GLP1s (a reduction of 11mmol/mol in HbA1c and a reduction of 3% in weight after 6 months of treatment) but it could be argued that more attention should be paid to the impact of all therapies on parameters such as HbA1c and weight when deciding whether to continue treatment; GLP1s are no different in that respect.
HOME BLOOD GLUCOSE MONITORING
In line with previous guidance on home blood glucose monitoring (HBGM), NICE states that this should primarily be offered if people are on treatment which carries the risk of hypoglycaemia – i.e. sulfonylureas, metiglinides or insulin. It is advised that particular consideration is given to the DVLA guidance on driving and hypoglycaemia risk when deciding whether to offer HBGM.9 It could also be argued that the same attention should be paid to this guidance when considering which treatment options to use. However, another group of people who might be considered for HBGM are women who are pregnant or who may be planning a pregnancy. This latter group will be cared for by the primary health care team so this is something general practice nurses may need to consider. A possibly more mystifying recommendation is to consider short term HBGM for people taking oral steroids. Bearing in mind that the main indication for HBGM is that something can be done in respect of any changes that are seen, this recommendation will need to be amended to suit the patient and the duration and dose of oral steroid treatment. It’s unlikely that NICE envisaged us all giving HBGM equipment to people with diabetes and COPD who are having a 5 day course of 40mg of prednisolone (as per the REDUCE study10) for an exacerbation. An important recommendation from NICE regarding HBGM is that an annual assessment of monitoring knowledge and skills should be carried out annually along with a check of the equipment being used. Although many GPNs will be doing this already this should be documented as having been done as it is not necessarily included in the diabetes template.
MANAGING CARDIOVASCULAR RISK
The guidelines encourage clinicians to link into other NICE guidance publications to access further information about reducing cardiovascular disease (CVD) risk. Specific guidelines mentioned are included in the resources below with links to the appropriate sections of the NICE website. Several areas have been updated in the past 12-18 months and are worth reviewing: examples include the guidance on lipid management and chronic kidney disease,11,12 both of which have been updated relatively recently and both of which have a significant impact on diabetes care.
Blood pressure management is another important component of reducing CVD risk and the effect of blood pressure lowering has been linked to better overall outcomes.13 This guidance reiterates the importance of treating blood pressure with an ACE inhibitor as these drugs offer cardioprotective and renoprotective benefits.14 However, the guidelines then go on to suggest that if additional treatment is required (as it often is in all cases of hypertension) a calcium channel blocker or a thiazide (or thiazide-like) diuretic should be used. This is at slight odds with the current NICE guidance for hypertension,14 which advises adding in a CCB as a second line drug and then a diuretic as a third option. In reality, drug choices will be made based on the individual patient, along with any co-morbidities and preferences so this is a minor discrepancy. The guidelines remind clinicians that the use of an ACE inhibitor with an angiotensin receptor blocker is not appropriate. This is in line with previous evidence that this ‘dual blockade’ of the renin angiotensin aldosterone system increases side effects without improving outcomes.15
It has been some time since the MHRA warned against the use of aspirin in primary prevention of CVD, including in people with diabetes.16 This advice is repeated in the guidelines, saying that neither aspirin nor clopidogrel should be prescribed in people with diabetes unless they have a diagnosis of CVD.
OTHER ADVICE
As well as the recommendation not to start metformin if the eGFR is less than 30ml/min, NICE also reminds people to consider reducing the dose in those with an eGFR of 45ml/min or less. This is because of the putative risk of lactic acidosis, although this is now known to be less common than has previously been thought.18 Advice is also given to avoid pioglitazone if there is macroscopic haematuria which has not been investigated and to avoid it in people with a history of bladder cancer. This suggests that NICE still has concerns about pioglitazone and bladder cancer despite research that refuted this.19
Although discussion of erectile dysfunction (ED) is no longer a requirement for QOF, NICE recognises the importance of this condition and its potential to cause significant morbidity. The guidelines therefore recommend that all men are given the opportunity to discuss ED and that appropriate investigations, treatment and referral are offered accordingly.
SPECIALIST INPUT
There is a certain lack of conformity when it comes to different localities’ approaches to when ‘specialists’ should be involved in diabetes care, or indeed what constitutes a specialist. Obviously no-one should be working outside of their competency and GPNs working in the often intricate field of diabetes care would be well advised to ensure that they have recognised and accredited training in this subject and are up to date with recent developments (see resources section below).
NICE makes some suggestions as to when expert opinion may be needed and these situations include:
- When there is a discrepancy between HbA1c measurements and other glucose measurements which might be taken
- Pregnancy
- When using a GLP1 with insulin
Other reasons for referring patients include complex cases where an expert opinion is needed.
CONCLUSION
In summary, then, the new NICE guidelines for managing type 2 diabetes reflect a proactive response to the criticisms levelled at the draft guidance and now encourage a patient-centred approach, which recognises the need for tailored targets and treatments. This is something to be celebrated and recognised by health care professionals and it is time to move away from the old structure of a ‘one size fits all’, ‘metformin – sulfonylurea – insulin’ approach that has been rolled out in the past. There are clinical, ethical and medicolegal reasons for involving patients in their care and offering them choices which recognise and respond to the heterogeneity of how they present. The guidance offered is very much in line with the NMC Code of Conduct and GPNs should be able to implement this guidance with confidence.
REFERENCES
1. NICE NG28. Type 2 diabetes in adults – management, 2015 https://www.nice.org.uk/guidance/ng28
2. Nursing and Midwifery Council. The Code. Professional standards of practice and behaviour for nurses and midwives, 2015 http://www.nmc.org.uk/standards/code/
3. Bostock-Cox B. The Nursing and Midwifery Code: Making it work for you and your patients. Practice Nurse 2015;46(04):34-37 http://www.practicenurse.co.uk/index.php?p1=articles&p2=1128
4. NHS England. Personalised care and support planning handbook: The journey to person-centred care, 2015. https://www.england.nhs.uk/wp-content/uploads/2015/01/pers-care-guid-core-guid.pdf
5. eMC Repaglinide, 2014 https://www.medicines.org.uk/emc/medicine/24638
6. NICE NG17. Type 1 diabetes: diagnosis and management – insulin delivery, 2015 http://www.nice.org.uk/guidance/ng17/chapter/1-Recommendations#insulin-delivery
7. Valentine V. The Role of the Kidney and Sodium-Glucose Cotransporter-2 Inhibition in Diabetes Management. Clinical Diabetes 2012;30(4):151-155
8. Zinman B et al. Empagliflozin, cardiovascular outcomes and mortality in type 2 diabetes N Engl J Med 2015 DOI: 10.1056/NEJMoa1504720
9. DVLA. At a glance guide to the current medical standards of fitness to drive, 2015. https://www.gov.uk/government/publications/at-a-glance
10. Leuppi JD et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA 2013;309(21):2223-31.
11. NICE CG181. Lipid modification, 2014 http://www.nice.org.uk/guidance/cg181/evidence/cg181-lipid-modification-update-full-guideline3
12. NICE CG182. Chronic kidney disease in adults, 2014 https://www.nice.org.uk/guidance/cg182
13. UKPDS. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes BMJ 1998;317:703
14. NICE CG127. Hypertension guideline, 2011 https://www.nice.org.uk/guidance/cg127
15. The ONTARGET investigators. Telmisartan, ramipril or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547–59.
16. MHRA. Aspirin not licensed for primary prevention of thrombotic vascular disease, 2009 http://www.mhra.gov.uk/Safetyinformation/DrugSafetyUpdate/CON087716
17. Misbin RI. The Phantom of Lactic Acidosis due to Metformin in Patients With Diabetes. Diabetes Care 2004;27(7):1791-1793
18. Ryder RE. Pioglitazone has a dubious bladder cancer risk but an undoubted cardiovascular benefit. Diabet Med;2015:32(3):305-13.
Related articles
View all Articles