Diabetes masterclass: Optimising therapies for type 2 diabetes
Beverley Bostock-Cox
Beverley Bostock-Cox
RGN, MSc, QN
Nurse Practitioner Mann Cottage Surgery Moreton in Marsh
Clinical Lead Education for Health Warwick
With so many treatment options available, choosing the best regime for your patients with type 2 diabetes can be challenging – but it is vital that glycaemic control is maintained
In a previous article on preventing the complications of diabetes, we discussed the importance of treating risk factors such as hyperglycaemia, hypertension and dyslipidaemia.1 In this article we look specifically at the drugs used to minimise risk focusing primarily on those used to improve glycaemic control. There is a plethora of medication available these days to treat raised blood glucose levels and while this allows us to offer a wider choice of therapeutic options to patients, it can be a challenge keeping up with the options available and understanding the pros and cons for each drug. Standard therapies, such as metformin, sulfonylureas (SUs) and insulin, have been joined over the years by pioglitazone, the DPP4 inhibitors, the GLP1 agonists, and SGLT2 inhibitors, each with key indications and contraindications for their use. So how can clinicians find the best fit for each patient that they see?
OBJECTIVES
After reading this article you should be able to:
- Understand the basic mode of action of drugs used to treat hyperglycaemia
- Evaluate the appropriateness of different drugs for different patients
- Recognise the role that national and local guidelines have to play in the decision making process
- Engage in meaningful discussions with patients about the options available to them and support them to make the right choice for them
- Consider the relative cost of drugs when prescribing (either directly, as a non-medical prescriber, or alongside a GP)
As previously discussed, around 10% of the total NHS budget goes on treating type 2 diabetes (T2D) with most of it being spent on treating the vascular complications of the condition.2 The risk of these complications can be minimised by appropriate lifestyle and pharmacological interventions aimed at improving blood glucose levels, blood pressure and the lipid profile. Lifestyle interventions include healthy eating, maintaining an appropriate BMI and taking regular activity.3 Avoidance of smoking and keeping alcohol intake to within recommended levels is also important. People with T2D should understand that pharmacological management should complement lifestyle changes and is not a replacement for adopting a healthier lifestyle.
The pharmacological approach to lowering blood glucose levels has been the subject of two draft guidelines from NICE recently, the latest in June.4 Prior to this, the last set of guidelines was published in 2009. However, the long awaited draft update which was released in January 2015 was greeted with what can only be described as derision, due to its lack of patient focus and apparent ignorance of the possible implications of its recommendations on patients and clinicians alike.5 NICE retreated to review its guidance, resulting in an amended version which was put out for consultation in June 2015.4 The consultation period has just ended and the final version of the guideline has yet to be released, but like the first version, version 2 places considerable emphasis on the acquisition cost of the drugs used to control blood glucose.
When considering which drug is most appropriate for the person with type 2 diabetes, thought should be given to the mode of action, any side effects, any impact on renal function, potential effect on cardiovascular risk and the cost of the drug. Basic acquisition cost should only be considered if all other elements of the drugs being considered are equal – something which is often not the case, either between classes or within classes. For example, it is important to consider the possible side effects of medication used to treat diabetes along with the cost of treating these side effects, such as hypoglycaemic events – ‘hypos’.
PROS AND CONS OF TREATMENT OPTIONS
When considering the main classes of drugs used to treat T2D, it is important to address the issues mentioned above.
Metformin
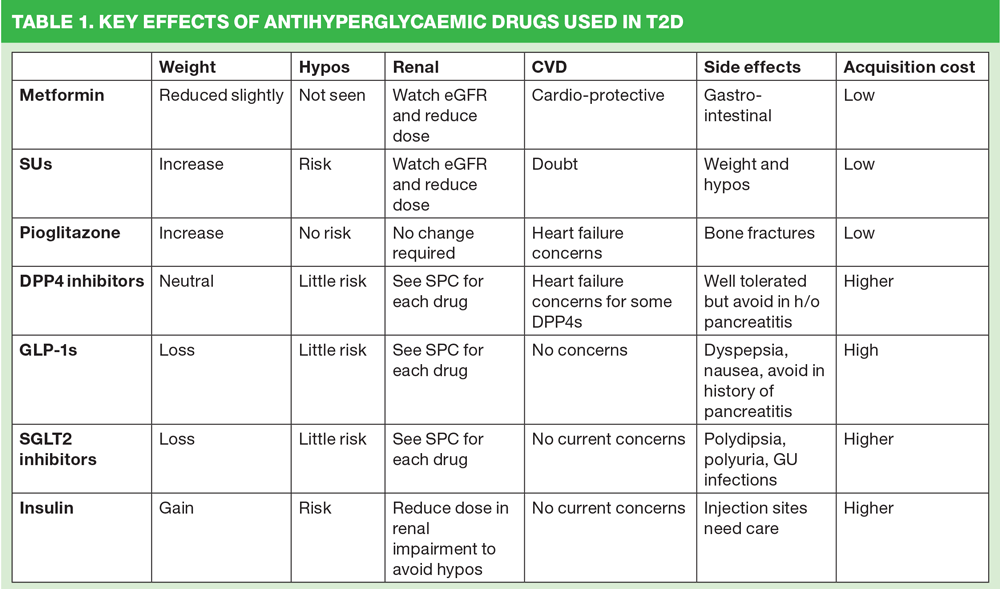
In most cases, the first line drug recommended to treat T2D is the biguanide, metformin. Metformin works by reducing glucose neogenesis and improving insulin sensitivity. These combined effects lower blood glucose levels without increasing the risk of hypoglycaemia. Metformin has been linked to a modest weight loss and is thought to be cardioprotective.6 All of these elements, along with a low acquisition cost make it an ideal first choice for most people with T2D. The gastrointestinal side effects of metformin (wind, diarrhoea) can be mitigated by starting on a low dose and gradually increasing over a period of weeks. If despite this approach metformin causes side effects which cannot be tolerated, modified release metformin can be given in a once daily dose of 1-2g. Renal impairment can impact on the use of metformin as there is a risk of lactic acidosis in people with an eGFR of less than 45 mls/min/1.73m2.7 However, it is thought that this risk is not as great as was previously assumed. How metformin fares in terms of the issues identified as being important is shown in Table 1.
Sulfonylureas
Sulfonylureas (SUs) are often used as the next step up after metformin although they do have some possible side effects, which mean that they are by no means suitable for all. However, their low acquisition cost means they remain a popular choice for many clinicians. They can lower blood glucose quickly and effectively by encouraging the pancreas to produce more insulin, albeit indiscriminately, thus leading to an increased risk of hypoglycaemia. The fast improvement in blood glucose levels can be useful for people suffering from the osmotic side effects of hyperglycaemia such as thirst and polyuria although this improvement is thought to be short-lived in many cases.8 There is much debate as to the cardiovascular effects of SUs with some studies showing a decrease in cardiovascular events while others showing an increase, probably linked to the risk of hypos.9 SUs can be used in renal impairment but consideration should be given to reducing the dose as reduced renal function can lead to higher levels of circulating drug thus increasing the risk of hypos. (Table 1)
Pioglitazone
Pioglitazone is the only surviving drug in the class known as thiazolidinediones (TZDs). Two other TZDs (troglitazone and rosiglitazone) have been withdrawn due to side effects. Given at a dose of 15-45mg daily, pioglitazone works by improving insulin sensitivity in a similar way to metformin. Pioglitazone has a low risk of hypoglycaemia but can cause weight gain, mainly due to fluid retention. It is therefore contraindicated in heart failure. It is also not suitable for people at risk of bone fractures, as it has been shown to increase this risk.10 Elderly people and those with an increased risk score for fragility fractures should avoid pioglitazone, therefore. When prescribing pioglitazone, which is subject to MHRA warnings, particular caution should be exercised in people who may be at risk of bladder cancer, although the latest research – published in the Journal of the American Medical Association just last month – suggests that the increase risk is ‘not statistically significant, although an increased risk could not be excluded.’11 (Table 1)
DPP4 inhibitors
Dipeptidyl peptidase 4 (DPP4) inhibitors are incretin-based therapies. Glucagon-like peptide-1 (GLP-1), which is released after eating and is therefore glucose dependent, allows insulin to normalise blood glucose levels after meals. Like metformin, GLP-1 also inhibits gluconeogenesis in the liver. GLP-1 activity is downregulated by an enzyme called dipeptidyl peptidase 4 (DPP4) so DPP4 inhibitors allow GLP-1 to go on working for longer, reducing glucagon release and elongating the activity of insulin while there is glucose available for it to work on.12 DPP4 inhibitors include the drugs sitagliptin 100mg, vildagliptin 5mg, saxagliptin 5mg, linagliptin 5mg and alogliptin 25mg. They can be used first line or can be used with other hypoglycaemic agents. Their glucose-dependent action means that they rarely cause hypoglycaemia unless used with other drugs such as SUs; they are weight neutral and most can be used in all stages of renal disease, although dose adjustment in renal impairment is required with the exception of linagliptin which is metabolised through the liver so needs no dose adjustment from normal renal function through to end stage renal disease. The doses of the other DPP4s should be reduced simply because poor renal function means the drug stays in the circulation for longer so lower doses are all that is needed to achieve the same effect. (Table 1)
Glucagon-like peptide-1s (GLP-1s)
The injectable GLP-1 analogue therapies mimic the action of naturally occurring GLP-1 but unlike naturally occurring GLP-1, they are also able to withstand the action of DPP4. The GLP-1 mimetics currently available include standard exenatide (Byetta) 5-10mcg bd, long acting exenatide 2mg (Bydureon) once weekly, liraglutide (Victoza) 0.6-1.2mg once daily, lixisenatide (Lyxumia) 10-20mcg once daily and duraglitide (Trulicity) 0.75mg and 1.5mg, which is another once weekly option. As well as prolonging the action of insulin and suppressing glucagon activity, GLP-1s also delay gastric emptying making people feel fuller for longer and also affect the satiety centre to reduce appetite.13 Both of these actions can help with weight loss.8 NICE states that GLP-1s are indicated in people with a BMI of 35+ although they can also be used in people with lower BMIs if there are significant co-morbidities.8 (Table 1)
GLP-1s with insulin
Most GLP-1s (Bydureon excepted) can be used in combination with insulin to reduce insulin requirements while optimising glycaemic control and minimising hypo risk and weight gain.14 Recently a combination product containing a basal insulin with a GLP-1 has been launched. This new product, Xultophy, contains insulin degludec and liraglutide. In trials it led to a reduction in HbA1c of over 1.8% in a year.15
Sodium-glucose cotransporter 2 (SGLT2) inhibitors
Sodium-glucose cotransporter 2 (SGLT2) include dapagliflozin 10mg once daily, canagliflozin (100mg increasing to 300mg once daily) and empagliflozin 25mg. They can be used as monotherapy or in combination with insulin or most other oral hypoglycaemic agents. SGLT2 inhibitors block the reabsorption of glucose from the renal system into the body and instead allow glucose to be excreted in the urine. This action leads to both improved glycaemic control and weight loss.16 The glycosuria seen with SGLT2 inhibitors can increase the risk of genitourinary infections although generally these infections occurred just once, usually in the first year of treatment and were mild to moderate in severity.17 The mode of action means that good renal function is usually required when using these drugs so they are therefore best initiated early – for example at step 2. However, the draft NICE guidance does not give them prominence, a situation which deserves to be challenged. Thirst and polyuria are known side effects of SGLT2 inhibitors but hypoglycaemia has not been thought to be an issue with these drugs; however, recent reports of diabetic ketoacidosis (DKA) in people taking SGLT2 inhibitors have been a cause for concern.18 The incidence of DKA is still being monitored and clinicians have been advised to remain vigilant to any cases and to report them via the yellow card system. There is nothing in their mode of action that makes SGLT2 inhibitors likely to cause DKA, which makes these episodes (which remain very rare) perplexing. (Table 1)
Insulin
Insulin can be used at any stage when treating T2D – as monotherapy from diagnosis, right through to when triple therapy with other drugs has not worked. Insulin can also be used as a ‘rescue’ therapy for people who have symptomatic hyperglycaemia until this resolves.4 Once glycaemic control has been restored, the use of insulin therapy can be reviewed. Although insulin can help to significantly improve glycaemic control it is also known to increase the risk of hypoglycaemia and weight gain. Furthermore, home blood glucose monitoring is essential, adding to the patient’s burden and increasing the cost to the NHS. When adding insulin to other therapies, clinical judgement should be exercised to determine whether other drugs should be stopped or doses reduced. (Table 1)
EVALUATING THE APPROPRIATENESS OF DIFFERENT DRUGS FOR DIFFERENT PATIENTS: CASE STUDIES
First intensification
It is important to evaluate the appropriateness of different drugs for different people, in the light of individual circumstances. Look at the short case studies below: these patients are all on metformin 2g and need to step up treatment. The different therapies have been discussed with the each individual and they are ready to make a decision. In your opinion, which drug fits each person best?
Steven age 42; BMI 42; eGFR 64 mls/min/1.73m2; works shifts as an electrician on the railways
High BMI suggests the need for a drug which will impact on weight as well as glycaemia; important to avoid hypos and weight gain. Consider GLP1 or SGLT2 – patient choice re oral or injectable; if injectable chosen, discuss once weekly v once daily options. Consider costs of all options. No need for home blood glucose monitoring (HBGM).
Carol age 78; BMI 26; eGFR 55 mls/min/1.73m2; retired, lives alone, drives to and from her daughter’s home 80 miles away every weekend
Older person so be careful to avoid hypos, especially as still driving. BMI in overweight category so could have an SGLT2 (but note eGFR); DPP4 inhibitor would work well here. No need for HBGM.
Rashpal age 34; BMI 33; eGFR 90 mls/min/1.73m2; maître d’hôtel in 5 star restaurant; polyuria and thirst
Younger man, obese but normal renal function. Busy life, needs to be on top of his game; around food a lot. Osmotic symptoms. SU may be appropriate here – short term until control improves. Need for HBGM. Consider SGLT2 or GLP-1 once control and symptoms improve. No need for HBGM once SU stopped.
Mohammed age 57; BMI 24; eGFR 74 mls/min/1.73m2; works as a consultant in A&E
Normal-increased BMI (consider ethnicity) and normal renal function; responsible job and shift work. Consider pioglitazone or DPP4. No need for HBGM.
Second intensification
At the time of writing the current draft NICE guidelines suggest that if HbA1c rises to 58mmol/mol on two drugs, triple therapy should be considered. NICE recommends metformin, pioglitazone and an SU or metformin, an SU and a DPP-4 inhibitor. The need for insulin should also be borne in mind. Bearing in mind the issues identified around SUs and pioglitazone above, the decision regarding appropriate treatment should be made based on the individual being treated and the risk:benefit ratio of the various drugs.
THE ROLE OF GUIDELINES
As already noted, the NICE draft guidelines have come in for severe criticism from people working in the field of diabetes care. The final version, which is due out very soon, will hopefully reflect these concerns and will be more in line with the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) guidelines, which recognise the need for tailored therapy regimens.19 Both national and local guidelines will usually contain a comment to the effect that nothing within the guidelines replaces clinical judgement and this is something which prescribers should recognise. A recent article on the NMC Code of conduct identified how following guidelines without thinking them through could cause nurses to be in breach of the Code.20 Local guidelines will often be based on using drugs with the lowest acquisition cost, assuming that many drugs have a ‘class effect’, i.e. that because they belong to the same family, they all have the same effect. We only have to look at the case of the TZDs to see this is not so – only pioglitazone lives to tell the tale. Recent issues around heart failure admissions in people using DPP4 inhibitors also suggest that there are inherent differences in drugs within the class. The SAVOR-TIMI study showed an increase in heart failure admissions in people on saxagliptin,21 whereas TECOS offers some reassurance that sitagliptin does not have the same effect.22 It may be somewhat foolish to make assumptions about molecularly distinct drugs within classes by saying they all have the same effects and side effects. Non-medical prescribers should be wary of being led automatically down this route.
SUPPORTING PATIENTS
It has been said many times (but perhaps adhered to less often) that there should be ‘no decision about me without me’.23 General practice nurses do not need to be experts in diabetes to be able to understand the key indications for the drugs mentioned above and to understand when drugs might also be contraindicated – or even just less suitable for the individual patient. Once people have had the opportunity to discuss the effects and side effects of the range of therapies they can be referred on, as necessary, to someone who has the relevant expertise and qualifications to prescribe. As the patient’s advocate, each nurse has a role to play in ensuring that the 4 Ps of the NMC Code24 are followed:
- Prioritise people
- Practise effectively
- Preserve safety
- Promote professionalism and trust
Ensuring that people with diabetes have tailored treatment which optimises outcomes and minimises risk will meet these 4 Ps.
REFERENCES
1. Bostock-Cox B. Preventing the complications of diabetes Practice Nurse 2015;45(7):18-22. http://www.practicenurse.co.uk/index.php?p1=articles&p2=1170
2. HSCIC. National Diabetes Audit 2012-13, 2015 http://www.hscic.gov.uk/searchcatalogue?productid=16971&q=%22National+diabetes+audit%22&sort=Relevance&size=10&page=1#top
3. Tuomilehto J, Lindstrom L, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance N Engl J Med 2001;344:1343-50.
4. NICE. Type 2 Diabetes draft guidelines, 2015 http://www.nice.org.uk/guidance/gid-cgwave0612/resources/type-2-diabetes-draft-guideline-nice2.
5. O’Hare JP, Miller-Jones D, Wasim H, et al.The new NICE guidelines for type 2 diabetes: a critical analysis. Br J Diabetes Vasc Dis 2015;15. http://diabetologists-abcd.org.uk/bjdvd/NICE_Diabetes_Editorial_2015.pdf
6. UK Prospective Diabetes Study Group: Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865
7. Misbin RI. The phantom of lactic acidosis due to metformin in patients with diabetes. Diabetes Care 2004;27(7):1791–3
8. NICE CG87. The management of type 2 diabetes, 2009. https://www.nice.org.uk/guidance/cg87
9. Phung OJ, Schwartzman E, Allen RW, et al.
Sulphonylureas and risk of cardiovascular disease:
Systematic review and meta-analysis. Diabet Med 2013;30(10):1160–71
10. Riche DM, King ST. Bone loss and fracture risk associated with thiazolidinedione therapy.Pharmacotherapy 2010;30(7):716-27.
11. Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA 2015;314(3):265-277
12. White JR. Dipeptidyl Peptidase-IV Inhibitors: Pharmacological Profile and Clinical Use. Clinical Diabetes 2008;26(2):53-57
13. Garber AJ. Long-Acting Glucagon-Like Peptide 1 Receptor Agonists: A review of their efficacy
and tolerability. Diabetes Care 2011;34(Suppl 2):S279-S284
14. Cowan J, McKay G, Fisher M. GLP-1 receptor agonists and insulin: Where are we now? Practical Diabetes 2012;29(9):351–352a
15. EMC. Xultophy, 2015 https://www.medicines.org.uk/emc/medicine/29493
16. Valentine V. The Role of the Kidney and Sodium-Glucose Cotransporter-2 Inhibition in Diabetes Management. Clinical Diabetes 2012;30(4):151-155
17. Bailey CJ, Gross JL, Pieters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: A randomised, double-blind, placebo-controlled trial. Lancet 2010;375(9733):2223–33
18. Brooks M. SGLT2 Inhibitor Diabetes Drugs May Cause Ketoacidosis: FDA, 2015. http://www.medscape.com/viewarticle/844754
19. Inzucchi SE, et al. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes http://care.diabetesjournals.org/content/38/1/140.extract
20. Bostock-Cox B. The Nursing and Midwifery Code: making it work for you and your patients. Practice Nurse 2015;45(4):34-37. http://www.practicenurse.co.uk/index.php?p1=articles&p2=1128
21. Scirica BM, et al. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. N Engl J Med 2013;369:1317-1326. http://www.nejm.org/doi/full/10.1056/NEJMoa1307684
22. Green JB, et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. NEJM 2015;373:232-242 http://www.nejm.org/doi/full/10.1056/NEJMoa1501352
23. Department of Health. Equity and excellence: Liberating the NHS, 2010. https://www.gov.uk/government/publications/liberating-the-nhs-white-paper
24. Nursing and Midwifery Council. The Code: Professional standards and behaviour for nurses and midwives, 2015. http://www.nmc-uk.org/standards/code
Related articles
View all Articles