A practical guide to introducing and titrating GLP-1 therapies
Beverley Bostock RGN MSc MA QN ANP in long-term conditions, Mann Cottage Surgery President-Elect Primary Care Cardiovascular Society
Practice Nurse 2025;55(5):26-30
Recent guidelines on obesity and the draft NICE guidance on type 2 diabetes mean that general practice nurses are more likely to be prescribing GLP-1 RAs or helping patients to get the best from their medications – so here is some practical advice on how to do so
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) belong to a class of medication that has been used in the management of type 2 diabetes (T2D) for decades, following many years in development.1 A recent addition to this stable of medication is tirzepatide which combines a GLP-1 RA with a glucose-dependent insulinotropic polypeptide (GIP) receptor agonist. Until recently, NICE only recommended the use of these medications after other therapies had been tried.2 NICE also stated that people needed to achieve certain targets for their HbA1c and weight to be allowed to continue them.However, in the draft update published in August,3 NICE has proposed bringing its guidance closer to the evidence-based recommendations from the American Diabetes Association and European Society for the Study of Diabetes (ADA/EASD) guidance.4 A key reason for this is because of the emerging evidence for their additional cardioprotective benefits and capacity to support weight loss.Indeed, the weight loss benefits have ensured their place in the management of obesity, with NICE publishing guidance on this topic earlier this year.5 Primary care clinicians are likely to be prescribing more GLP-1 RAs as a result of these guidelines and so this article aims to support general practice nurses (GPNs) to understand how to counsel people who are on these medications in order to optimise outcomes.
After reading this article, GPNs should be able to:
- Recognise what GLP1-RAs are and how they work
- Evaluate the similarities and differences between the key products
- Understand dose schedules and possible side effects and know how to reduce their impact
- Advise on lifestyle interventions to support the use of GLP-1 therapies
- Apply the practical tips to ensure that the medication is appropriate for each individual
WHAT ARE GLP-1 RAS AND WHAT IS THEIR MODE OF ACTION?
GLP-1 RAs and the GLP-1/GIP RA combination, tirzepatide, are incretin-based therapies. GLP-1 is an incretin hormone secreted by intestinal L-cells in response to the intake of food, particularly carbohydrate.1 GIP is a hormone that stimulates insulin release from the pancreas in response to raised blood glucose levels.It also plays a role in regulating energy balance which helps to support weight reduction when combined with a GLP-1 RA.6 The GLP-1 RAs and the GLP-1/GIP RA bolster the physiological effects of the body’s naturally occurring incretin hormones.The key actions of these therapies are:7
Enhanced secretion of insulin from the β-cells of the pancreas in response to the presence of glucose in the gastrointestinal (GI) tract
Reduced secretion of glucagon from the of α-cells of the pancreas, decreasing the release of glucose stores from the liver
Delayed gastric emptying, slowing the absorption of food from the GI tract to reduce portion consumption and making people feel fuller sooner during a meal and for longer after eating.This is one of the key reasons for the weight loss seen with the use of these therapies.
Appetite regulation via the action on the satiety centre in the hypothalamus, leading to reduced calorie intake and resulting in weight loss.
Cardiovascular benefits (for some products)due to vasodilatory, natriuretic, anti-inflammatory properties plus reductions in blood pressure and improvements in lipid profile.8,9
Renal benefits: Several GLP-1 RAs, including dulaglutide, liraglutide and semaglutide have been shown to slow the progression of chronic kidney disease, primarily through improved glycaemic control, blood pressure reduction, weight loss, and direct renal effects.10
Hypoglycaemia: very low risk of hypoglycaemia due to the mode of action; risk increases when combined with sulfonylureas or insulin.
DIFFERENTIATING BETWEEN THE PRODUCTS
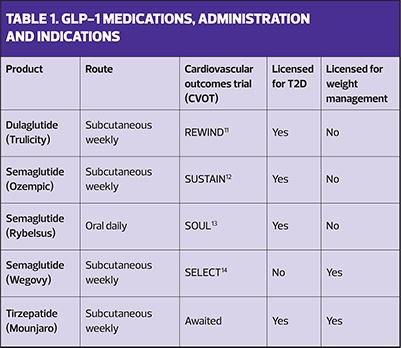
The draft NICE guidelines are out for consultation and await ratification, not least with respect to which GLP-1-based treatments might be prioritised but the key products that are currently available are described here and in Table 1.
Short-acting subcutaneous GLP-1 RAs include exenatide and lixisenatide.These have to be injected daily and have failed to show cardiovascular benefits, so are no longer routinely recommended.
Short-acting oral GLP-1 RA Semaglutide is available as a tablet, taken once daily on an empty stomach with the patient taking nil else by mouth for at least 30 minutes afterwards to ensure that the medication is absorbed effectively.
Long-acting subcutaneous GLP-1 RAs include liraglutide, dulaglutide and semaglutide, all of which are injected once weekly.
Long-acting subcutaneous GLP-1/GIP RA known as tirzepatide given once weekly
If weight loss is a key target, tirzepatide andsemaglutide 2.4 mg have been shown to be the most effective, with tirzepatide demonstrating optimal benefits in a recent head-to-head trial.15 The drugs with proven cardiovascular benefits include liraglutide, semaglutide and dulaglutide.The cardiovascular outcomes trial for tirzepatide is anticipated later this year.16
DOSE SCHEDULES
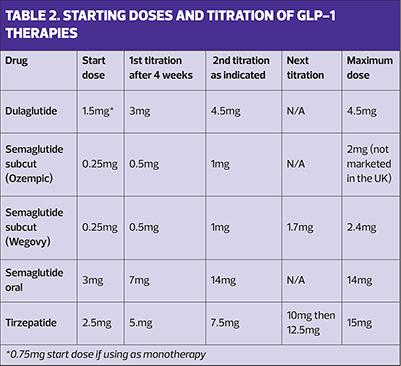
To minimise gastrointestinal (GI) side effects, GLP-1-based therapies should be started at a low dose for a month before uptitrating to the most effective dose for that individual, depending on the indication for use – T2D or obesity. (See Table 2).
Injection technique
Patient information leaflets and videos are available from the companies that make the GLP-1-based therapies. (See Resources)
SIDE EFFECT PROFILE AND MANAGEMENT
The side effect profile of these drugs is similar for all products and relate to the mode of action. The most commonly reported side effects are gastrointestinal and include nausea, vomiting, diarrhoea or constipation. However, these are generally short-lived although they may recur when doses are uptitrated. Less common side effects include pancreatitis and gall bladder disease. These medications can generally be used in the elderly, and in renal and hepatic impairment without the need for dose adjustment, although individual summaries of product characteristics should be consulted before prescribing. These can be found at https://www.medicines.org.uk/emc.
These drugs should be avoided in pregnancy and breastfeeding, and advice should be given about using extra barrier methods of contraception and adjusting doses of oral hormone replacement therapy when prescribing tirzepatide.17,18 These restrictions do not apply to semaglutide or other GLP-1 therapies. On the advice of an anaesthetist, GLP-1 and GLP-1/GIP RAs should be stopped before a general anaesthetic as the slowed gastric emptying may increase the risk of aspiration.19
Retinopathy may deteriorate when glucose levels reduce quickly, as may be seen with GLP-1 therapies and with insulin. Care should be taken to review the results of the latest retinopathy screen to determine whether treatment with a GLP-1 is appropriate. Retinopathy is not a contraindication, but caution is advised, especially if the grade is reported to be 2 or above.
PRACTICAL PRESCRIBING TIPS
It is important to remember that an understanding of the advantages and disadvantages of different therapies is essential, even for non-prescribers.As the patient’s advocate, the GPN needs to be able to talk people through the different devices and why one medication might be better than another, based on the relevant indication and desired outcome.
Consider the indication for treatment with a GLP-1 based therapy. In T2D, they will be used for glycaemic control, with consideration given to the additional weight loss and/or cardiovascular benefits. The draft NICE update for T2D currently recommends semaglutide for diabetes, although it is very possible that tirzepatide will be added to the final update. The ADA/EASD guidance indicates that both of these drugs are highly effective for both glycaemic control and weight loss.4 Although normally added second or third line, they are also licensed to be used first line if necessary. In some cases, the cardiovascular risk reduction seen in people with T2D will be a key deciding factor for the use of these therapies, independent of glycaemic control.
When prescribing for obesity, local guidance should be consulted. Subcutaneous semaglutide at the maintenance dose of 2.4 mg or tirzepatide uptitrated to a maximum dose of 15mg can be prescribed for weight management in people with or without diabetes. It is important to note that the licenced indication for treating obesity is in people with a BMI ≥30 kg/m2, or ≥27 kg/m2 with comorbidities. However, NICE and NHS England recommend a rollout programme targeting those with a BMI >40 and four or more weight-related comorbidities, initially as a way of managing demand.5
Whatever the indication for prescribing, it is essential that clinicians and patients alike understand that these drugs should only be used as part of a comprehensive approach including dietary interventions, physical activity, and behaviour change.
There are some general rules for all of these medications. The aim is to start at a low dose and uptitrate gradually to reduce GI side effects and increase adherence. The target dose will depend on whether the medication is being used for T2D or for weight management. Ensure people have been trained to use the device or are aware of how to take oral semaglutide i.e., take on an empty stomach, with a sip of water only, and have nothing else by mouth for 30 minutes afterwards. It is also important to monitor progress through reductions in HbA1c for T2D, and through weight loss for obesity management. In people with T2D, both targets will be relevant. Clinicians should review tolerability and support patients to reduce side effects. This might include giving advice about eating smaller meals and avoiding greasy or spicy foods and/or alcohol. Ginger tea, anti-nausea wrist bands or over the counter travel sickness medications (which may also cause drowsiness) may help some people. Diarrhoea medication or laxatives can also be bought from pharmacies. People should be reassured that although side effects might occur when initiating or increasing doses of GLP-1-based therapies, they usually improve with time. It is also important to remind people that as well as having a smaller appetite, they might also notice that they feel less thirsty, so they should be reminded to maintain their fluid intake. This is especially relevant if people are also taking SGLT2 inhibitors, where volume depletion has been linked to an increased risk of diabetic ketoacidosis.
- Highlight CV and kidney benefits beyond glucose lowering.
- Injection technique: Reassure about needle size and simplicity of devices.
- Lifestyle integration: Reinforce that GLP-1 therapy complements, not replaces, healthy habits.
Use with other medication in T2D
All GLP-1 RAs and GLP-1/GIP RAs can be used with other diabetes medications includingmetformin and SGLT2 inhibitors.However, they should not be used with DPP-4 inhibitors (gliptins) as they are both incretin-based therapies so there is no advantage to using them both. DPP-4 inhibitors also have no impact on weight, or cardiovascular or renal outcomes. When using GLP-1 RAs or GLP-1/GIP RAs with sulfonylureas or insulin, there is an increased risk of hypoglycaemia so reduced doses of these two drugs should be considered.
Lifestyle interventions to optimise the use of GLP-1 RA therapies
People should be advised about setting realistic weight loss goals. The media may well have created unsustainable expectations about how much weight they will lose and how quickly this may happen.Gradual, sustained changes are better.According to some of the studies, approximately 1-4% of the starting weight will be lost in the first month, going up to 22% after 18 months but this varies enormously and will depend on multiple factors. A regular loss of 0.5 – 1.0 kg per week is ideal.
It is important to remember that the weight loss that occurs with these therapies has also been linked to sarcopenia.20 Loss of muscle mass is a concern, especially in frail or elderly populations. This may be reduced by ensuring that people have an adequate intake of protein and that this is prioritised when preparing and consuming meals. Maintaining or increasing physical activity levels may also help to preserve muscle mass.
Although the role of GLP-1 RAs and GLP-1/GIP RAs in cardioprotection is becoming increasingly clear, it is important to take a holistic approach to CVD risk reduction through lifestyle changes, blood pressure management and lipid-lowering therapy as indicated.
CONCLUSION
GLP-1 RA-based therapies represent one of the most significant advances in diabetes management in decades. However, their benefits extend well beyond glycaemic control, and the impact on weight reduction has led to some of these treatments being licensed, and endorsed by NICE, for the management of obesity. Along with these indications, some members of the class have demonstrated cardiovascular benefits, and they also have the potential for offering additional benefits for kidney disease, metabolic liver disease and heart failure. GPNs need to have a robust understanding of the mechanisms, efficacy, safety, and practical use of these treatments in order to support people with shared decision-making.
RESOURCES
Information, including injection technique, for patients and healthcare professionals
- Lilly. How to use the Mounjaro® (tirzepatide) KwikPen. https://medical.lilly.com/uk/products/answers/how-to-use-the-mounjaro-tirzepatide-kwikpen-219072
- Lilly. Your guide to Mounjaro® https://uk.lilly.com/metabolic/assets/pdf/mounjaro-patient-booklet.pdf
- NovoNordisk. Welcome to Ozempic® (semaglutide injection)https://www.ozempic.co.uk/patientresources.html (https://www.ozempic.co.uk/patientresources.html)
- Diabetes UK. Rybelsus – uses, how it works, side effects https://www.diabetes.org.uk/about-diabetes/looking-after-diabetes/treatments/tablets-and-medication/semaglutide/rybelsus
- NovoNordisk. Welcome to Wegovy® (semaglutide injection) https://www.wegovy.co.uk/home.html
- Lilly. Trulicity® (dulaglutide): How to use the pen https://medical.lilly.com/uk/products/answers/trulicity-dulaglutide-how-to-use-the-pen-116202
References
- Friedman JM. The discovery and development of GLP-1 based drugs that have revolutionized the treatment of obesity. Proc Natl Acad SciUSA 2024;121(39): e2415550121. https://doi.org/10.1073/pnas.2415550121
- NICE NG28. Type 2 diabetes in adults; 2022 https://www.nice.org.uk/guidance/ng28
- NICE. Type 2 diabetes in adults: management Draft for consultation; 2025. https://www.nice.org.uk/guidance/gid-ng10336/documents/450
- Davies MJ, Aroda VR, Collins BS, et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022;45(11):2753–2786. https://doi.org/10.2337/dci22-0034
- NICE NG246. Overweight and obesity management; 2025. https://www.nice.org.uk/guidance/ng246
- Nauck MA, Meier JJ. Incretin hormones: Their role in health and disease. Diabetes Obesity Metab 2018;20(Suppl 1):5–21. https://doi.org/10.1111/dom.13129
- Clark L. GLP-1 receptor agonists: A review of glycemic benefits and beyond. J Am Acad Physic Assist 2024;37(4):1–4. https://doi.org/10.1097/01.JAA.0001007388.97793.41
- Liu QK. Mechanisms of action and therapeutic applications of GLP-1 and dual GIP/GLP-1 receptor agonists. Front Endocrin 2024;15:1431292. https://doi.org/10.3389/fendo.2024.1431292
- Ma X, Liu Z, Ilyas I, et al. GLP-1 receptor agonists (GLP-1RAs): cardiovascular actions and therapeutic potential. Int J Biol Sci 2021;17(8):2050–2068. https://doi.org/10.7150/ijbs.59965
- Michos ED, Tuttle KR. GLP-1 Receptor Agonists in Diabetic Kidney Disease. Clin J Am Soc Nephrol (CJASN) 2021;16(10), 1578–1580. https://doi.org/10.2215/CJN.18771220
- Rodriguez PJ, Goodwin Cartwright BM, Gratzl S, et al. Semaglutide vs Tirzepatide for Weight Loss in Adults With Overweight or Obesity. JAMA Intern Med 2024;184(9):1056–1064. https://doi.org/10.1001/jamainternmed.2024.2525
- Gerstein HC, Colhoun HM, Dagenais G R, et al for the REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394(10193):121–130. https://doi.org/10.1016/S0140-6736(19)31149-3
- Nauck MA, Quast DR. Cardiovascular Safety and Benefits of Semaglutide in Patients With Type 2 Diabetes: Findings From SUSTAIN 6 and PIONEER 6. Front Endocrinol 2021;12:645566. https://doi.org/10.3389/fendo.2021.645566
- McGuire DK, Marx N, Mulvagh SL, et al and the SOUL Study Group (2025). Oral Semaglutide and Cardiovascular Outcomes in High-Risk Type 2 Diabetes. New Engl J Med 2025;392(20):2001–2012. https://doi.org/10.1056/NEJMoa2501006
- Lincoff AM, Brown-Frandsen K, Colhoun HM, & SELECT Trial Investigators. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. New Engl J Med2023;389(24), 2221–2232. https://doi.org/10.1056/NEJMoa2307563
- Nicholls SJ, Bhatt DL, Buse JB, et al for the SURPASS-CVOT investigators. Comparison of tirzepatide and dulaglutide on major adverse cardiovascular events in participants with type 2 diabetes and atherosclerotic cardiovascular disease: SURPASS-CVOT design and baseline characteristics. Am Heart J 2024;267, 1–11. https://doi.org/10.1016/j.ahj.2023.09.007
- Faculty of Sexual and Reproductive Healthcare. GLP-1 agonists and oral contraception; 2025. https://www.cosrh.org/Common/Uploaded%20files/documents/CEU-statement-GLP-1-agonists-and-contraception.pdf
- Gray S, Hazell T, Price L, Thomas L. Injectable weight loss drugs, contraception and HRT; 2025. https://www.pcwhs.co.uk/_userfiles/pages/files/resources/glp1_contraception_hrt_article.pdf
- MHRA. GLP-1 and dual GIP/GLP-1 receptor agonists: potential risk of pulmonary aspiration during general anaesthesia or deep sedation; 2025. https://www.gov.uk/drug-safety-update/glp-1-and-dual-gip-slash-glp-1-receptor-agonists-potential-risk-of-pulmonary-aspiration-during-general-anaesthesia-or-deep-sedation
- Memel Z, Gold SL, Pearlman M, et al. (2025). Impact of GLP- 1 Receptor Agonist Therapy in Patients High Risk for Sarcopenia. Curr Nutr Rep 2025;14(1):63. https://doi.org/10.1007/s13668-025-00649-w
Related articles
View all Articles