Masterclass: using combination inhalers in COPD
Beverley Bostock-Cox
Beverley Bostock-Cox
RGN, BSc, MSc
Nurse Practitioner Mann Cottage Surgery Moreton-in-Marsh
Clinical Lead, Education for Health, Warwick
Since the NICE guidance on COPD was updated four years ago, the evidence and thinking behind this important condition have evolved, and there is a great deal to consider when selecting therapies to offer patients the best possible outcomes
he NICE guidelines for chronic obstructive pulmonary disease (COPD) were updated in 2010 and included an algorithm for using inhaled therapies.1 In the four years since this update was published, the options and evidence have changed. This article aims to focus on the use of combination inhalers (inhaled corticosteroids [ICS] combined with long acting beta2 agonists [LABA]) in the management of COPD and will cover the drugs, devices, indications and potential side effects of the treatments available. There will also be a brief discussion on newer treatment options joining the market.
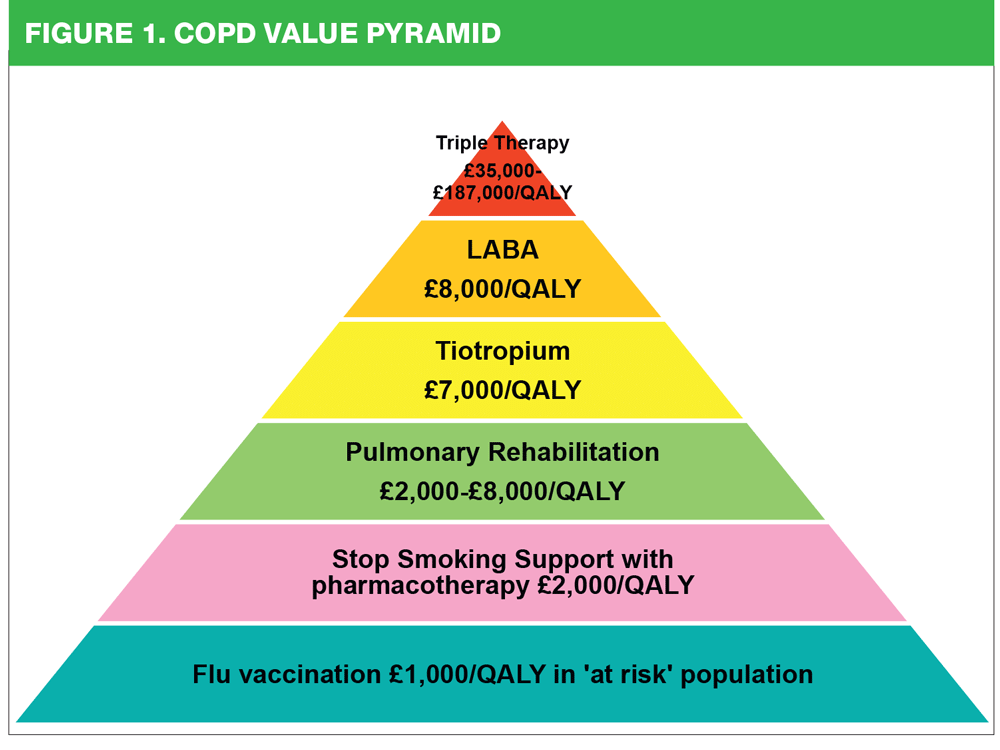
The NICE guidelines stress the importance of using both pharmacological and non-pharmacological interventions to improve outcomes for people with COPD.1 The London Respiratory Network (LRN) has developed a pyramid diagram that shows the cost effectiveness of the range of interventions for COPD (Figure 1). This underlines the importance of using the full complement of interventions in the most effective way.
The most cost effective interventions include immunisation against flu, the use of pulmonary rehabilitation services and offering appropriate smoking cessation support. Combination inhalers used with long acting muscarinic antagonists (LAMA) as triple therapy are at the top of the pyramid, suggesting that care should be taken to ensure that these treatments are reserved for use in patients where the benefits are greatest. So how can we identify these individuals?
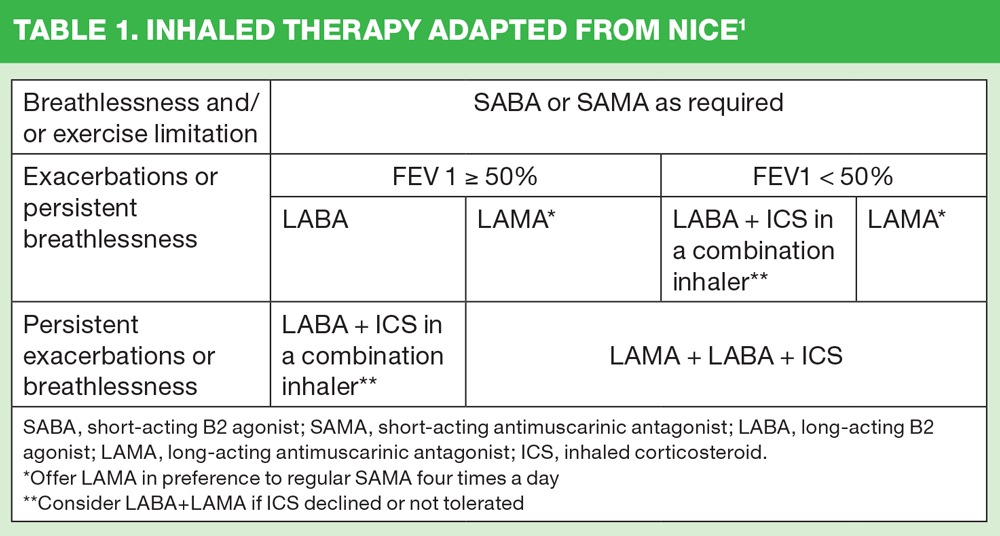
The current NICE guidelines algorithm sets out the recommendations for inhaled treatment options (Table 1). Three of these refer to combination inhalers, with their use suggested for people with an FEV1 equal to or greater than 50% predicted if they have persistent breathlessness or exacerbations, or in people with an FEV1 of less than 50% predicted if they have ‘persistent exacerbations’ (Table 1).
Yet the evidence for the use of combination inhalers is not as strong as some might think, although it is strongest for people who suffer persistent exacerbations i.e. two or more exacerbations in a year.2,3 Not only will these patients benefit more, they will also have an improved risk: benefit ratio when it comes to considering the potential side effects of the ICS doses that are sometimes used in COPD.
Evidence suggests that many exacerbations go unreported,4 and it is therefore essential that clinicians ask patients about any exacerbations when they attend for review. The NICE guidelines recommend that exacerbation frequency is recorded at the annual review, but this is not always included in COPD templates, so may be missed. Knowing the frequency of ‘flare ups’ can help the clinician tailor treatment. The usual definition of an exacerbation is an acute and sustained increase in symptoms (such as cough, breathlessness and sputum production) which is above and beyond the individual’s normal symptoms and which requires an adjustment to be made to the regular treatment regimen.5
Not all patients with COPD suffer regular exacerbations. The Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-Points (ECLIPSE) study showed that although COPD exacerbations were increasingly seen as the patient’s disease worsened, the strongest predictor of future exacerbations was a previous history of exacerbation.6 This was observed across all patients, regardless of their disease severity. In those who did not exacerbate, the risk of exacerbating in the future was significantly less.
The ECLIPSE study confirms the growing realisation that COPD is a heterogeneous disease – in other words, it behaves in different ways in different people. This view that COPD sub-groups, or phenotypes exist has led to a developing interest in matching treatments to type. The evidence for using combinations in people with two or more exacerbations recognises that those who exacerbate are more likely to continue to exacerbate without treatment with a combination inhaler, whereas those whose phenotype is breathlessness will arguably be better served with bronchodilator therapy – an antimuscarinic agent or a beta2 agonist, or both.
THERAPY OPTIONS
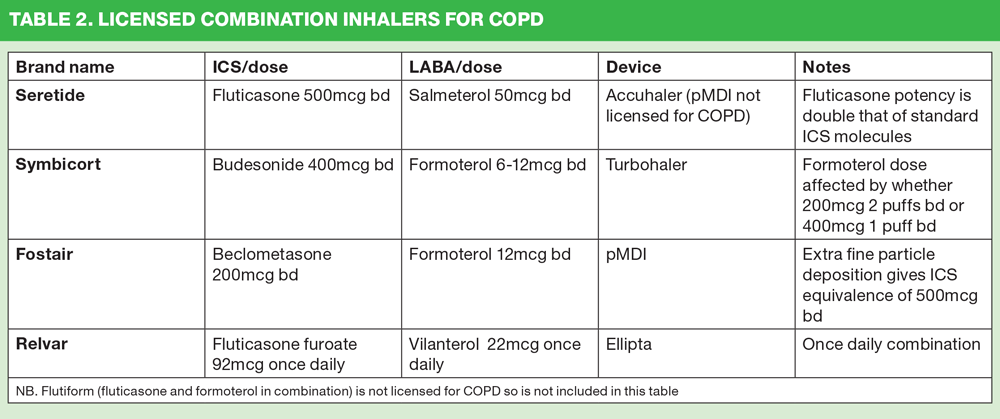
At the time of writing there are four licensed combination inhalers for COPD in the UK (Table 2).
There may be different reasons for choosing one product over another and they may change from patient to patient. In many instances the merits and challenges of the device may be a key consideration before the drug is chosen. No drug can work if it is not delivered to the lungs because the patient cannot use the inhaler. The importance of assessing inhaler technique and matching the device to the individual cannot be overestimated. Once the right device has been chosen, the drug available in that device can be considered in terms of its suitability. For combination therapies in COPD, there is a choice of a pressurised metered dose inhaler (pMDI) with Fostair, or a dry powder inhaler (DPI) with the other three products. Many people are anxious about using DPIs in people with COPD fearing that they will not have the required inspiratory flow rate to activate the device. However, in COPD the problem is usually difficulty exhaling rather than inhaling, and studies have confirmed that most people are able to inhale to the inspiratory flow rate required to activate a DPI.7,8
pMDIs are the most commonly prescribed device in the UK; paradoxically this is also the type of device that has been shown to have the highest level of incorrect use.9 Studies have confirmed that teaching people how to use a pMDI (especially with a spacer device) can result in correct technique,10 so time must be spent observing and objectively assessing inhaler technique before treatment begins and at every review. Asking patients is not enough: Basheti11 showed that when asked, 75% of patients being reviewed said they were confident about using their inhalers but only 10% actually demonstrated correct technique. However, in another study of health care professionals, only 7% actually prepared and used the device they were asked to demonstrate properly and showed the correct inspiratory flow rate for that device.12 Tools such as placebos with built in auditory inspiratory flow indicators (e.g. the Turbowhistle) or the In-Check device (including the new In-Check M™) can be useful aids. Training, and regular checks of inhaler technique, are as important for the health care professionals who teach and check patients’ techniques as they are for the patients!
The choice of inhaler may focus on the molecules in the inhalers. All four combinations contain different ICS molecules, as shown in table 2. There has been much recent discussion of the pros and cons of each ICS and debate about their relative risks and benefits.
Fluticasone propionate (FP) is a potent steroid molecule. It is highly lipophilic which allows it to ‘bed down’ into the tissue, facilitating prolonged action within the lungs. The 500mcg bd licensed dose of Seretide gives an equivalent dose of 2000mcg of a standard ICS molecule. High doses of ICS have been linked to potential side effects, including pneumonia and diabetes,2,13 and people who have been prescribed a high dose ICS (usually considered to be over 1000mcg daily) are advised to carry a steroid warning card at all times.14 The LRN has recently developed an advice sheet and a steroid card (see resources) to be given to people who are taking a dose of ICS equivalent to 1000mcg or more. However, their view is that rather than simply giving out these cards clinicians should perhaps be looking to ensure this dose is rarely prescribed. Although all ICS molecules used to treat COPD have been shown to increase the risk of pneumonia,15 some studies have suggested that FP carries an increased risk of pneumonia compared with other ICS molecules.16,17
Budesonide is a standard ICS molecule and at a daily dose of 800mcg is below the 1000mcg ‘ceiling’ suggested by the LRN. The beclometasone dipropionate (BDP) in Fostair is an extra-fine particle formulation that has the potential to improve deposition in the small airways.18 The fact that more drug can get down into the lungs means that although 400mcg is the prescribed daily dose for COPD, the effect is the same as 1000mcg of standard beclometasone. There is some debate as to whether this means that the Fostair ICS dose hits the 1000mcg ceiling or not. However, research into the effect of extra fine BDP suggests that adrenal suppression does not occur with this dose.19,20
Fluticasone furoate (FF) is a newer molecule in inhaler therapy, although it has been used for some time in nasal spray form for allergic rhinitis. The effect of FF has been shown to last longer so that a once daily dose is all that is needed. Relvar combines a once daily dose of 92mcg of FF with a once daily LABA, vilanterol 22mcg, to offer the first once daily combination inhaler. This dose is licensed for both asthma and COPD, with a higher dose option (184/22mcg) being available for severe asthma. Following its launch in the early part of 2014, concerns were expressed that the device had a blue top and that this, along with the name might confuse some people into thinking that this was their ‘reliever’ inhaler. This has led to some areas in England advising against its use in their guidelines, although the product is still licensed for use and can be prescribed if the clinician feels the benefits outweigh any concerns. The simple device along with the once daily dose might mean that it could be a suitable option for some, carefully selected, patients If it is thought that a patient would benefit from this product it is important that they are very clear that this is only used once daily – ‘once a day then put it away’ – to avoid any uncertainty.
The LABAs in the range of combination products include salmeterol, formoterol and vilanterol. The onset of action of these LABAs is fastest with formoterol, slowest with salmeterol with vilanterol sitting somewhere in the middle. Further details of the various products can be found at the electronic medicines compendium website, http://www.medicines.org.uk/emc/.
STEPPING DOWN IN COPD
It is important not to confuse stepping down ICS-based treatments for COPD with stepping down in asthma. Stepping down for COPD only happens when the decision is made to switch to a lower dose of treatment with the aim of reducing the steroid dose; in asthma, stepping down is recommended once full control has been established and the individual needs a lower dose of ICS to maintain control. If the patient with COPD is on a high dose ICS combination and it is thought that a lower dose is preferable then doses can be altered immediately without the concern about a ‘loss of control’ which we might bear in mind in people with asthma.
The primary function of the ICS/LABA inhaler in COPD is to reduce exacerbations and all of the licensed drugs and doses can be used to do this. Some patients with COPD may also have a reversible element (i.e. some asthma as well as COPD) but the doses of all of the licensed ICS/LABA products for COPD are suitable for treating asthma effectively too. Concerns about stepping down are often unnecessary, therefore. If a patient is on an ICS/LABA combination and a review of their records suggests that continued use of a combination inhaler may not be warranted, the dose should be stepped down gradually over a period of weeks or months and ongoing assessment and monitoring carried out to ensure that the patient remains stable off treatment. They may well need a LABA only (or potentially a LAMA) to improve symptoms and consideration should be given to adding in a long-acting bronchodilator once the combination treatment is stopped.
FUTURE DEVELOPMENTS
A new device offering the combination of budesonide and formoterol (equivalent to 400mcg and 12mcg respectively and prescribed as a twice daily dose) in a novel breath-actuated DPI will be available later this year and will offer another opportunity to match the device to the patient whilst keeping the dose of ICS below the 1000mcg ceiling. A new DPI version of Fostair will also be available in the future.
For those patients who do not need a combination ICS/LABA inhaler, there are some newer products available which aim to reduce symptoms of breathlessness and improve quality of life for people with COPD who do not suffer from exacerbations but where breathlessness is the main concern. Currently the NICE guidelines recommend the use of short acting bronchodilators (B2 agonists or anti-cholinergics/anti-muscarinic agents) until they are required regularly when a long acting version should be introduced.1 The guidelines also say that combinations of LABAs and LAMAs should only be used if the patient is unable or unwilling to take an ICS combination inhaler. However, the thinking around this guidance has changed significantly since the guidelines were issued and the benefits of dual bronchodilators have been demonstrated in several studies.21,22 On this basis, both once daily and twice daily LAMA/LABA bronchodilators are coming onto the market, including Anoro (umeclidinium and vilanterol 55mcg/22 mcg once daily) and Ultibro (indacaterol and glycopyrronium 85/43mcg once daily) with more to follow. In those patients who need symptom relief from breathlessness and where recurrent exacerbations are not an issue, these drugs may offer the hope of symptom relief and improved quality of life in the future. The prospect of triple therapy inhalers containing an ICS, LABA and LAMA is likely to become a reality before long.
In summary, combination ICS/LABA inhalers offer the opportunity to reduce the frequency of exacerbations in patients with COPD who have a history of recurrent flare ups. In view of the fact that these inhalers have potential risks as well as benefits, patients should be carefully selected to identify those where the risk: benefit ratio is greatest. Once patients have been identified as being suitable for treatment the best product should be selected from the range available, bearing in mind the patient’s ability to use the device and the type of medication and dose of ICS available. In this way, people with COPD can have treatment tailored to their needs, including smoking cessation support, pulmonary rehabilitation and pharmacological therapy, which together will offer them the best opportunity for improved outcomes.
REFERENCES
1. NICE (2010) Management of COPD in primary and secondary care http://www.nice.org.uk/guidance/CG101
2. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J, TORCH investigators: Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease N Engl J Med 2007, 356:775-789.
3. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta2 agonist in one inhaler versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 9. http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0014169/
4. Scullion J, Holmes S. Strategies to reduce COPD exacerbations Practice Nursing 2011;22(2):83–86
5. Rodriguez-Roisin R. Toward a Consensus Definition for COPD Exacerbations. Chest 2000;117:398-401
6. Hurst JR et al for the Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) investigators. Susceptibility to Exacerbation in Chronic Obstructive Pulmonary Disease N Engl J Med 2010;363:1128-1138
7. Malmberg LP et al. Inspiratory flows through dry powder inhaler in chronic obstructive pulmonary disease: age and gender rather than severity matters Int J Chron Obstruct Pulmon Dis. 2010;5:257-62.
8. Prime D et al. Comparison of inhalation profiles through a novel dry powder inhaler (nDPI) and lung function measurements for healthy subjects, asthma and Chronic Obstructive Pulmonary Disease (COPD) patients. Am J Respir Crit Care Med 2012 185: A2941.
9. Levy M et al. Asthma patients’ inability to use a pressurised metered-dose inhaler (pMDI) correctly correlates with poor asthma control as defined by the Global Initiative for Asthma (GINA) strategy: a retrospective analysis PCRJ 2013;22(4):406-411
10. Cambridge Consortium. Evaluation of Inhaler Technique Improvement Project, 2012 http://www.tvhiec.org.uk/wpcms/wp-content/uploads/120904-CIREM_ITIP_HIEC_Evaluation1.pdf
11. Basheti IA et al. Evaluation of a novel educational strategy, including inhaler-based reminder labels, to improve asthma inhaler technique. Patient Educ Couns 2008;72:26–33.
12. Baverstock M et al. Do healthcare professionals
have sufficient knowledge of inhaler techniques in order to educate their patients effectively in their use? Thorax 2010;65:A117-A118
13. Suissa et al Inhaled corticosteroids and the risks of diabetes onset and progression Am J Med 2010;123(11):1001-6
14. NICE. Clinical Knowledge Summaries: Corticosteroids – inhaled, 2010. http://cks.nice.org.uk/corticosteroids-inhaled#!scenariorecommendation:8
15. Price D et al. Risk to benefit ratio of inhaled corticosteroids in patients with COPD. PCRJ 2012;21:208-213
16. Janson C et al. Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting -2 agonist: observational matched cohort study (PATHOS). BMJ 2013;346:f3306 http://www.bmj.com/content/346/bmj.f3306 (note corrections)
17. Suissa S et al. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax 2013;68:1029–36.
18. Labiris & Dolovich. Pulmonary drug delivery. Part II: the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br J Clin Pharm 2003;56(6):600–612
19. Huchon G, Magnussen H, Chuchalin A et al. Lung function and asthma control with beclomethasone and formoterol in a single inhaler. Respir Med 2009;103(1):41-9
20. Poli G, Acerbi D, Novellaux F et al. Pharmacological and pharmacodynamics of a new beclometasone dipropionate and formoterol CFC-free fixed combination in healthy volunteers (Poster). Presented at: European Respiratory Society Congress. Munich, 2–6 September 2006
21. Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD Respiratory Medicine 2013;107(10):1538-46
22. Vogelmeier C et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol/fluticasone in patients with COPD (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respiratory Medicine 2013;1(1): 51-60
Related articles
View all Articles