Dual bronchodilators in chronic obstructive pulmonary disease
Beverley Bostock-Cox
Beverley Bostock-Cox
RGN, MSc, QN
Nurse Practitioner Mann Cottage Surgery Moreton in Marsh
Education Lead Education for Health Warwick
There has been much discussion about when and for whom we should use dual bronchodilators but some degree of confusion persists, not helped by conflicting national and international guidelines. Practice Nurse provides some clarity
Chronic obstructive pulmonary disease (COPD) is a condition which is defined by irreversible airflow obstruction and which in the western world is primarily due to smoking.1 The latest UK guidelines on managing COPD were published by the National Institute for Health and Care Excellence (NICE) in 2010,2 although more recently, the latest quality statements on COPD from NICE were published earlier this year (2016 ).3 The Global Initiative for Obstructive Lung Disease (GOLD) group produces annual guidelines, the latest of which was updated in 2016.1
There are some important differences in the suggested treatment options as defined by each of these guidelines, which have the potential to affect which drug therapy is used to treat patients. NICE’s focus on lung function as a deciding factor in treatment options (FEV1 more or less than 50% of predicted) is different from GOLD’s wider focus on symptoms and exacerbation risk. There is evidence to suggest that lung function often fails to correlate with symptoms,4 so a symptom-based approach or one aimed at reducing exacerbation risk could be considered to be more appropriate. Defining a patient’s COPD phenotype may help to identify the most appropriate therapy for them and lead to more people being treated with dual bronchodilators than has previously been the case. Interest in this approach to managing COPD has led to several dual bronchodilators being brought to the market.
By the end of this article you should be aware of:
- The GOLD ABCD algorithm as a method of categorising patients’ symptoms and exacerbation risk
- How to identify which patients are likely to benefit most from ICS/LABA versus single or dual long acting bronchodilation
- How to recognise each of the four dual bronchodilators currently available
- How to help patients to choose the most appropriate inhaler for them
AIMS OF TREATMENT
According to GOLD the two main aims of treating COPD are to reduce symptoms such as cough and breathlessness and also to reduce the risk of exacerbations, which are associated with worsening lung function and an increased risk of dying.2 Breathlessness is a key feature of COPD and most patients will experience this as part of their condition. As a result, most people with COPD will need a bronchodilator. The decision will need to be made on which option is most appropriate: a short and/or long-acting beta2 agonist and/or muscarinic antagonist. The main indication for inhaled steroids is in reducing exacerbation risk so these should be reserved for people who are most at risk of exacerbations.2
PHENOTYPES
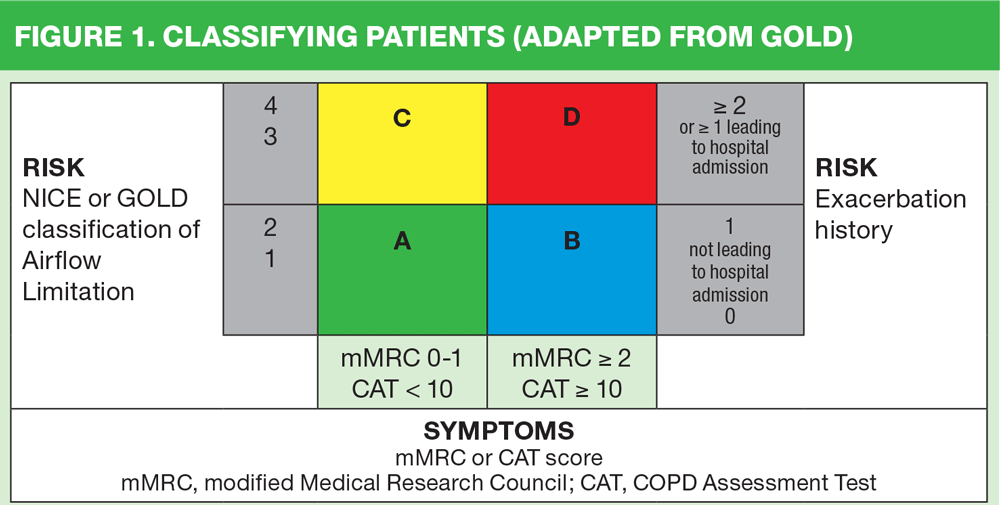
The GOLD guidelines contain an algorithm that allows a ‘phenotype’ to be identified by clinicians based on the patient’s symptom burden (along the x axis) along with exacerbation history (on the right hand y axis). This is known as the ABCD algorithm. Patients’ symptom scores are based on their modified Medical Research Council (MRC) dyspnoea score (European version, scored from 0-4 rather than the UK’s 1-5) or their COPD Assessment Test result (available at www.catestonline.org). Using this information along with the number of exacerbations suffered in the past 12 months, the patient’s category can be identified as A, B, C or D. (Figure 1)
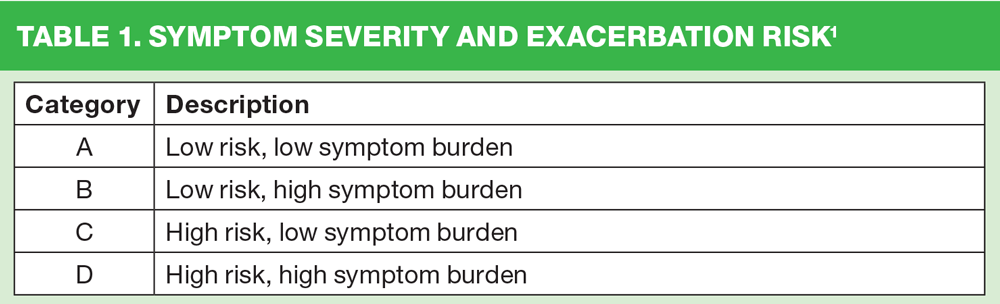
The category that the patient falls into indicates the severity of their symptom burden along with their risk of future exacerbations. (Table 1)
ICS/LABA v dual bronchodilation
The Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-Points (ECLIPSE) study showed that the patients most at risk of having exacerbations in the future are those who are already having exacerbations.5 This effect was seen across all patients, regardless of their disease severity. Those individuals having two or more exacerbations (or one exacerbation that was serious enough to require admission to hospital) are most likely to benefit from being treated with inhaled corticosteroids (ICS) combined with a long acting beta2 agonist LABA.6 Those who have no history of exacerbations had significantly less risk of exacerbating in the future and would have least to gain from an ICS/LABA.
This approach to ‘phenotyping’ people with COPD, along with the recognised risk of side effects from high dose ICS in particular,7,8 means that ICS/LABA combinations are no longer considered the most appropriate treatment for patients who do not exacerbate and that the use of long-acting bronchodilators may offer the best overall outcomes. This has led to an increased focus on the role of dual bronchodilation, using beta 2 agonist drugs combined with muscarinic antagonists to achieve this.
POSSIBLE TREATMENT CHOICES
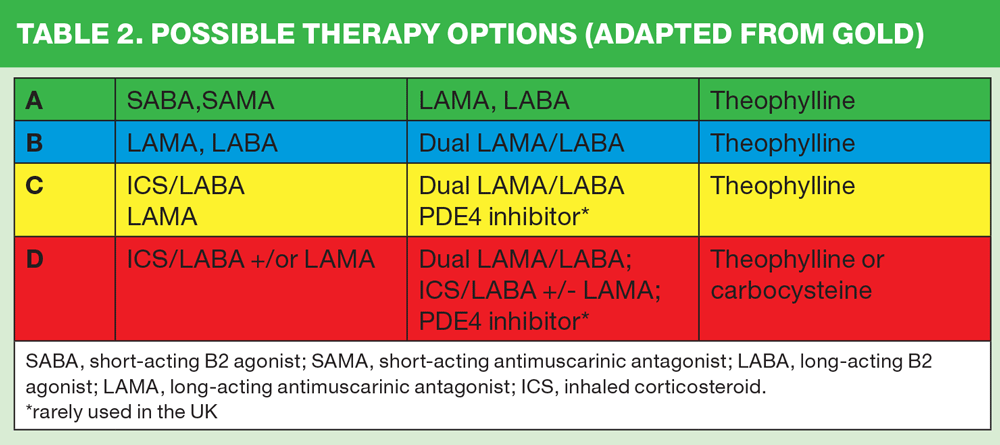
Once the patient’s symptom and exacerbations cores have been used to identify which section the patient belongs to, A, B, C or D, the first, second and third line approaches can be identified from the GOLD guidelines. (Table 2)
As can be seen from this table, long acting bronchodilators feature in all four categories, often as the first choice, with dual bronchodilators considered to be appropriate treatment for people in categories B, C and D.
This is different from the NICE guidelines which actively discourage the use of a LAMA and LABA together unless an ICS is not tolerated or is declined. (Table 3)
This approach is now considered to be outdated by many people as it could result in unnecessary use of an ICS/LABA. As an example, a patient with an FEV1 of 46% of predicted who complains of persistent breathlessness but who has no history of exacerbations could be identified by NICE as being suitable for treatment with a combination inhaler (ICS and LABA) whereas the GOLD guidelines would not suggest this as a first, second or even third line option. It is now thought that too many people with COPD are on inhaled corticosteroids who do not need them.9
THE RATIONALE FOR DUAL BRONCHODILATION
The GOLD guidelines state that dual bronchodilators should be considered as first line treatment for people in category B (heavy symptom burden with high mMRC or CAT scores but less than two exacerbations in the past 12 months) and as a second line option, added in to other therapies, for people in all other categories. This means that it is important the general practice nurses (GPNs) are aware of them and know when and why they should be used.
The two bronchodilators work in different ways. Beta 2 agonists increase levels of cyclic adenosine monophosphate (AMP) in the sympathetic nervous system leading to relaxation of bronchial smooth muscle and so aiding bronchodilation. Muscarinic antagonists prevent bronchoconstriction via the parasympathetic nervous system by inhibiting the binding of acetylcholine with muscarinic receptors on airway smooth muscle.2
These two complementary actions allow for maximum bronchodilation, as well as reduced lung hyperinflation in patients with COPD, thus alleviating shortness of breath.
PRODUCTS AVAILABLE
The dual bronchodilators are licensed for the relief of breathlessness in people with COPD. The potential for long acting bronchodilators to reduce exacerbations has been the subject of much discussion and further trial data in this area is due for release imminently. Although there is some evidence from existing studies using these drugs that they may reduce exacerbation risk, none are specifically licensed for this indication. Adverse drug reactions from these drugs are relatively few and include nasopharyngitis, headache, cough and dry mouth.
There are four dual bronchodilators currently available (Table 4).
Vilanterol and umeclidinium – dry powder inhaler (Anoro)
Anoro™ is a once daily dual bronchodilator containing the LABA vilanterol at a dose of 55mcg per puff and the LAMA umeclidinium at a dose of 22mcg per puff. The medication is delivered via the Ellipta™ dry powder inhaler device. Anoro can be used as a maintenance bronchodilator therapy to relieve symptoms in people with COPD. There are no restrictions for use in elderly patients or those with renal or hepatic impairment, although caution is advised in those with severe hepatic impairment as the drug has not been tested in this group. Studies carried out to assess the efficacy of Anoro on a range of parameters (lung function, quality of life and dyspnoea) showed clinically meaningful improvements when compared with either of the bronchodilators used alone. Interestingly, acute exacerbations were reduced by 20-30% in people taking vilanterol/umeclidinium compared with those taking one of the two components alone (i.e. not in combination) and by 50% when compared with placebo.
The device is loaded by sliding back the cap until a click is heard. At this point the drug is ready for inhalation. As with other dry powder inhalers, a fast and deep inhalation should be taken to optimise deposition.
Formoterol and aclidinium – dry powder inhaler (Duaklir)
Duaklir™ is the only twice daily dual bronchodilator. It contains the LABA formoterol at a dose of 12mcg and the LAMA aclidinium at 340mcg per puff. The medication is delivered via the Genuair™ dry powder inhaler device. There are no restrictions for use in elderly patients or those with renal or hepatic impairment. As with Anoro, studies carried out to assess the efficacy of Duaklir on lung function, quality of life and dyspnoea showed clinically meaningful improvements when compared with either bronchodilator used alone or versus placebo. Low resistance in the Genuair device means that patients COPD can reliably inhale their medication. It is worth asking people whether they have a preference for taking their long acting inhalers once or twice daily as some will prefer the convenience of a once daily approach while others might feel psychologically more comfortable ‘topping up’ twice daily.
Indacaterol and glycopyrronium – dry powder inhaler (Ultibro)
Ultibro™ is a once daily dual bronchodilator containing the LABA indacaterol 110mcg and the LAMA glycopyrronium 50mcg per puff. The medication is delivered via capsules using the Breezhaler™ dry powder inhaler device. There are no restrictions for use in elderly patients or those with mild or moderate renal or hepatic impairment. The FLAME study, due out imminently, is set to report on the impact of Ultibro on exacerbations.
Olodaterol and tiotropium – Soft Mist inhaler (Spiolto)
Spiolto™ is a once daily dual bronchodilator containing the LABA olodaterol 2.5mcg and the LAMA tiotropium 2.5mcg per puff, taken as 2 puffs once daily. The medication is delivered via the Respimat Soft Mist inhaler device. The device should be assembled before use and needs to be twisted in order to load each puff before inhalation via a soft mist which is released on depressing the activation button. There are no restrictions for use in elderly patients or those with mild or moderate renal or hepatic impairment.
Further information on each specific product can be found on the Electronic Medicines Compendium website: https://www.medicines.org.uk/emc/
SIDE EFFECTS OF LONG ACTING BRONCHODILATORS
Beta2 agonists can have an effect on cardiovascular parameters such as pulse rate and blood pressure and have also been known to increase the possibility of ECG changes being seen, although whether these changes are of any clinical importance is hard to assess. As with any other drug treatment, the risk: benefit ratio should be assessed for each individual case.
Muscarinic antagonists should be used with caution in people with a history of eye problems (notably glaucoma) and bladder outlet or prostate problems.
DEVICE CHOICE
Currently the licence for each of the dual bronchodilators is for relief of breathlessness symptoms through a similar mode of action. In many ways, then, the decision as to which treatment to use may come down to patient preference (e.g. for a once daily or twice daily treatment option or preference for a certain device), their ability to use the device and, to a lesser extent, the recommendations on local formularies, which may be based on acquisition cost. GPNs should bear in mind the 4 Ps of the NMC Code10 when supporting patients in making the correct inhaler choice. Formularies are written for populations and while they may be helpful for providing a ‘rule of thumb’ they do not and cannot provide the personalised approach which is the cornerstone of patient centred care. It is the role of the health care professional to translate generic guidelines into tailored and personalised care.
- Prioritise people
- Practice effectively
- Preserve safety
- Promote professionalism and trust10
CONCLUSION
Deciding how best to treat an individual patient’s COPD will depend on the severity of symptoms such as breathlessness, along with the frequency of exacerbations. These two elements will allow the categorisation of each individual into GOLD A, B, C or D. Only patients in category D are very likely to need an ICS/LABA although these combination inhalers may also be appropriate for category C patients. Dual bronchodilators have a key role to play across all four categories, however. Whichever class the patient is assigned to, device choice is an important consideration when choosing within the therapy class. There are four dual bronchodilators and the most appropriate treatment should be selected based on the patient’s ability to use the device, frequency of dosing and any other appropriate considerations including any degree of renal impairment.
This maximises the possibility of ensuring that patients with COPD get the best possible treatment while minimising all avoidable treatment related risks.
REFERENCES
1. Global Initiative for Obstructive Lung Disease. COPD guidelines, 2016. http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/
2. NICE CG 101. Chronic obstructive pulmonary disease in over 16s: diagnosis and management, 2010. https://www.nice.org.uk/guidance/cg101
3. NICE QS10. Chronic obstructive pulmonary disease in adults (Quality Standard), 2016. https://www.nice.org.uk/guidance/QS10/chapter/List-of-quality-statements
4. Westwood M, Bourbeau J, Jones PW, et al. Relationship between FEV1 change and patient-reported outcomes in randomised trials of inhaled bronchodilators for stable COPD: a systematic review. Respir Res 2011;12:40.
5. Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010;11:122.
6. Spencer S, Evans DJ, Karner C, et al. Inhaled corticosteroids versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011;(10) CD007033
7. Crim C, Calverley PM, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J 2009;34(3):641–647.
8. Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med 2010; 123(11):1001-6.
9. White P, Thornton H, Pinnock H, et al. Overtreatment of COPD with Inhaled Corticosteroids – Implications for Safety and Costs: Cross-Sectional Observational Study. PLoS ONE 2013;8(10):e75221. doi:10.1371/journal.pone.0075221
10. NMC. Code of Conduct, 2015 https://www.nmc.org.uk/standards/code/
Related articles
View all Articles