Understanding heart failure
JOANNE LOADES
JOANNE LOADES
RN MSc
Independent Nurse Consultant & Specialist in Cardiovascular Disease
Heart failure presents a significant burden to patients, their families and the wider health and care systems. Optimised treatments, supported self-management and regular review can both extend and improve quality of life; and primary care nursing teams can add tremendous value throughout the patient pathway
Heart failure is a complex clinical syndrome of signs and symptoms suggestive of an inefficiently pumping heart, confirmed with objective evidence of a structural or functional abnormality of the heart.1 Heart failure sits within the spectrum of cardiovascular disease and the most common cause of heart failure is coronary heart disease (CHD).1 Many patients who develop heart failure will have a history of myocardial infarction (MI) in the past though there are multiple other causes, many of which are avoidable, such as uncontrolled hypertension.2
The detection, diagnosis and management of heart failure is increasingly led by primary care. Innovations in service, along with improvements in treatment have influenced the changing face of heart failure over recent years. Despite these developments heart failure remains a potentially devastating condition for patients and their families, that can, however, be significantly improved by effective management and support from general practice nurses.
WHERE ARE WE NOW?
In 2015-16 the average practice prevalence of heart failure was 0.8%.3 This has remained fairly consistent over the past ten years, though a large population study in 2014 suggested the number of people living with heart failure in the UK was around 1.4% of the total population.4 Though people with heart failure are being diagnosed earlier, and at a younger age, it seems there are still people out there to be found in order to enable them to access to life improving treatments.5
The burden on the health system is significant with around 1–2% of the total NHS budget estimated to be spent on heart failure; 60–70% of this is related to the costs of hospitalisation.5 As with most long-term conditions, priorities of care should support early diagnosis and intervention with optimised, evidence based treatments, along with supported self-management and effective monitoring, in order to maintain patients in their own homes and avoid hospital admission where possible.6
WHERE CAN NURSES ADD MOST VALUE?
There is clear evidence that heart failure specialist nurses improve outcomes in patients with heart failure, but nurses working in general practice also have an important role in supporting patients and enabling self-management throughout the care pathway.7
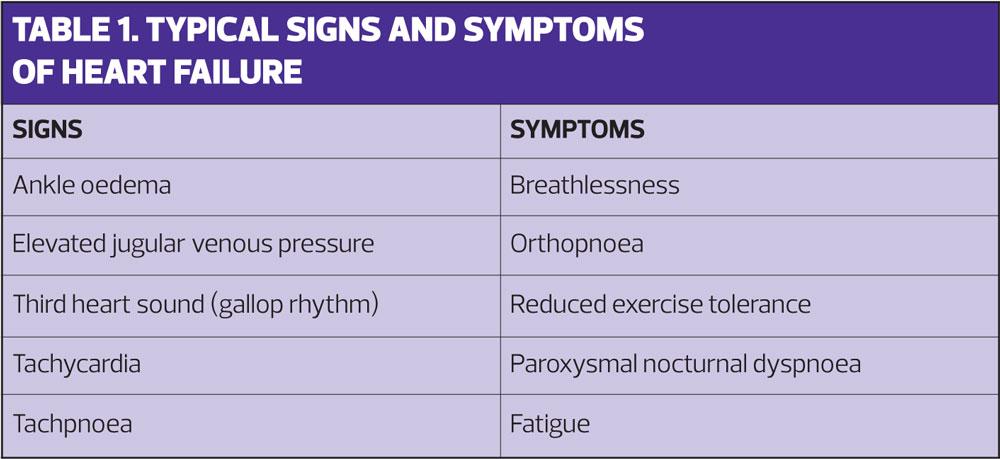
In terms of diagnosis, practice nursing teams are ideally placed to detect early signs and symptoms of heart failure (Table 1), given that they are largely responsible for the routine monitoring of patients with coronary heart disease and hypertension, the underlying causes in the majority of cases. Nurses may be responsible for the initiation and titration of medicines, or work in partnership with GP’s and specialist nurses to ensure patients are on the optimal therapy and have access to appropriate information to help them manage their condition. Routine monitoring and support are essential components of heart failure care throughout the trajectory of the disease and given that heart failure remains a life-limiting condition, the palliative care needs of the patient and their family should be considered by all involved in their care.6
TYPES OF HEART FAILURE
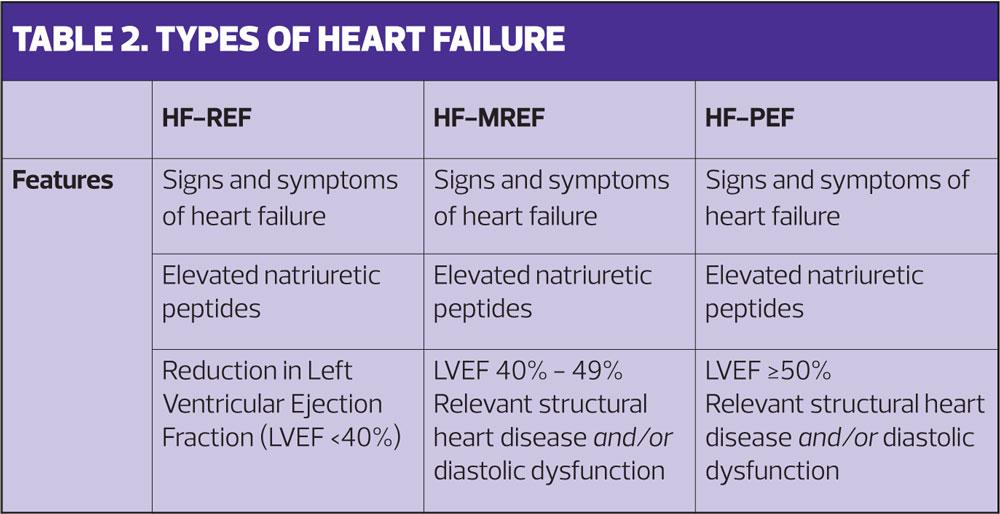
Heart failure is largely described as two-types, heart failure with reduced ejection fraction (HF-REF) and heart failure with preserved ejection fraction (HF-PEF). In recent years a third descriptor-type of heart failure with moderately reduced ejection fraction (HF-MREF) has been added. (Table 2) Signs and symptoms may be similar for all types so the diagnostic category is determined by imaging of the heart, usually echocardiography. The treatments for each type differ so it is really important that a correct diagnosis is made.6
Ejection fraction
The term ejection fraction refers to the amount of blood the left ventricle pumps out with each contraction. In the normal heart the ejection fraction will be between 50% and 70% and this will provide adequate circulation to meet the body’s needs. Heart failure results when the heart is unable to eject enough blood to fully meet the body’s needs. This will either be due to the left ventricle being impaired and therefore ejecting a reduced volume of blood, or the heart muscle becoming thickened and stiff and unable to relax and fill properly during the diastolic phase. The term preserved ejection fraction indicates that a normal percentage of the volume may be ejected, but impaired filling of the ventricle means that the actual volume is reduced. This is also known as diastolic dysfunction. The third term of moderately reduced ejection fraction usually indicates elements of both features.8 At least half of all patients with heart failure will have a reduced ejection fraction.8
DIAGNOSING HEART FAILURE
When clinical suspicion of heart failure arises it is important to act quickly. Given the similar mortality rates between heart failure and some cancers, it is appropriate that NICE recommend that those at highest risk undergo specialist assessment within two weeks of presentation.6 Unfortunately, despite advances, there are still major challenges and variation in access to diagnostic tests for heart failure across the country (as reported by the All Party Parliamentary Group [APPG] on Heart Disease,9) and in some areas, this remains an aspirational target.
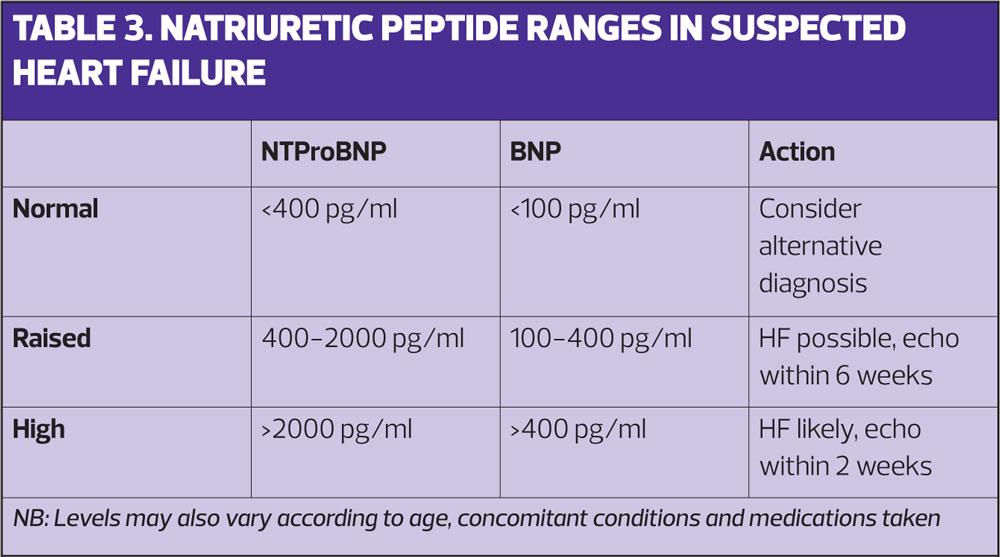
The NICE diagnostic pathway recommends that, following detailed history taking and a clinical examination, patients who have previously had an MI are referred directly for echocardiography.6 In patients without previous MI a blood test should be performed to measure natriuretic peptides. In most cases this will be a NTproBNP test (N-terminal pro b-type natriuretic peptide) that will, if raised, support the diagnosis of heart failure and if normal, make the diagnosis of heart failure unlikely.6 As can be seen in Table 3 the reference ranges vary according to the type of test used so it is important to be aware of which is in use in your local area.
The role of BNP/NTproBNP testing is well established in the UK as a rule out test that reduces the number of normal echocardiograms performed. Both NICE,6 and the APPG on Heart Disease,9 include this as a ‘must do’ for commissioners to make available in primary care. Following full assessment with echocardiography the confirmed diagnosis should be made and the patient added to the practice heart failure register.
RECOMMENDED INTERVENTIONS
Recommended treatment options will vary according to the type of heart failure diagnosed, and from a pharmacological perspective strategies will consist of treatments to improve mortality and morbidity and those that improve symptoms.
HF-REF
Standard Therapy
The most widely documented, evidence-based, pharmacological treatments are indicated in HF-REF and consist of a triple-therapy approach.
For many years angiotensin-coverting enzyme inhibitors (ACE-I) such as ramipril and perindopril have been the mainstay of heart failure treatment and should be initiated and up-titrated in all patients unless contraindicated.6 An angiotensin receptor blocker (ARB), such as candesartan, should be used where patients are unable to tolerate an ACE-I.6 A cardioselective beta-blocker, such as bisoprolol or carvedilol, should be added to the ACE-1 and again this should be up-titrated to an optimal dose. The third drug that is now recommended as standard therapy for all symptomatic patients with HF-REF is a mineralocorticoid receptor antagonist (MRA) such as spironolactone or epleronone.8 Given the possibility of side effects, particularly gynaecomastia in men, epleronone is probably the kinder choice in this instance. All of these drugs act on the neuro-hormonal pathways that are activated in heart failure, and which result in symptom-inducing effects such as fluid and sodium retention and tachycardia.8
Other options
A fairly new therapeutic class of drug for patients who remain symptomatic, and have been hospitalised with acute decompensation within the past year, is the angiotensin receptor neprilysin inhibitor (ARNI), sacubitril. The combination of sacubitril and the ARB, valsartan, currently available as Entresto®, can replace the ACE-1 in the treatment plan. This switch needs to be done carefully and is usually undertaken by a specialist or under a shared care agreement as there is a risk of symptomatic hypotension and other side effects.8
For heart rate reduction, ivabradine can be used to slow the heart in symptomatic patients with a heart rate over 70bpm despite optimal beta blocker therapy. Ivabridine is only effective in patients with a normal heart rhythm as it acts directly on the sinus node, the heart’s natural pacemaker, to slow down the heart rate.
HF-PEF and HF-MREF
In the case of HF-PEF and HF-MREF there unfortunately remains a dearth of evidence for treatments to improve morbidity and mortality. Treatment strategies are largely based on management of concomitant conditions, and risk factors such as hypertension and diabetes.6,8 Such individuals should, however, usually be seen by a cardiologist to review potential underlying causes. European Society of Cardiology (ESC) guidelines also suggest that since many of these patients are elderly and highly symptomatic with a poor quality of life an important aim of therapy may be to alleviate symptoms and improve quality of life.8
DIURETICS
Diuretic therapy is widely used and has an essential role in the treatment of fluid retention in patients with heart failure.8 However, it must be stressed that the role of diuretics is to ease symptoms rather than improve outcomes in heart failure as there are limited data on their ability to improve heart failure related morbidity and mortality.10
It is recommended that diuretics are used to achieve and maintain euvolaemia in patients – also known as ‘dry weight’ – at the lowest possible dose.10 This means that they should be titrated up when fluid is in excess and reduced, or even stopped, after restoration of euvolaemia to avoid dehydration, hypotension and renal dysfunction.10 The most commonly used loop diuretic is furosemide and, to be most effective, this should be used in conjunction with close monitoring of weight, fluid restriction where necessary, and avoidance of salt and non-steroidal anti-inflammatory agents where possible.10
DEVICE THERAPIES
Patients with significantly impaired cardiac function who remain symptomatic despite optimal medial therapy, and/or those who are at risk of life-threatening arrhythmias, may be candidates for device therapy.8 Implantable cardioverter defibrillators (ICD) and cardiac resynchronization therapy (CRT) have an important role to play in such patients and can both extend, and improve quality of life.11 It is really important that patients who may benefit from these devices and who are eligible have these options made available to them; and it useful for primary care practitioners to be familiar with the referral criteria to ensure appropriate patient’s benefit.
SELF-MANAGEMENT
Self-management in heart failure should be underpinned by education and support to help the patient undertake daily activities to monitor and maintain their condition. Good self-management is linked to reduced mortality and morbidity, including fewer hospital admissions.12 The ESC recommends a number of steps for self-management of heart failure. (Box 1)
Fluid management
It can be extremely useful for patients with heart failure to monitor their weight on a regular, ideally daily basis. Rapid weight gain is associated with excess fluid and could lead to an episode of acute decompensation if not promptly managed. Patients should be advised to report a sudden unexpected weight gain of >2kg in 3 days so diuretic therapy can be initiated or increased to remove the excess fluid.11 In most cases patients with mild to moderate heart failure will not require routine fluid restriction but this could be considered in patients with more severe symptoms.11
Salt
Limitation of salt intake is generally recommended in the entire population and is of particular relevance to people with heart failure. Given that sodium levels determine body fluid volume it makes sense that patients with heart failure should avoid excess salt.11 It is useful to discuss salt intake in the presence of heart failure with a particular emphasis on hidden salts, such as those in ready meals and processed foods.
Physical activity and exercise
A key symptom of heart failure is breathlessness and limitation of exercise capacity so it can seem counter-intuitive to recommend an increase in physical activity. There is clear evidence, however, that physical activity is beneficial to all patients with heart failure unless they are in The New York Heart Association (NYHA) class IV or have other limiting co-morbidities.11
The benefits of physical activity are extensive and include increased exercise capacity, improved QOL, reduction in depression and anxiety and overall improved survival.11 NICE recommends that patients with heart failure are referred to a structured rehabilitation and exercise programme but, in reality, availability of these across the country in variable.
MONITORING
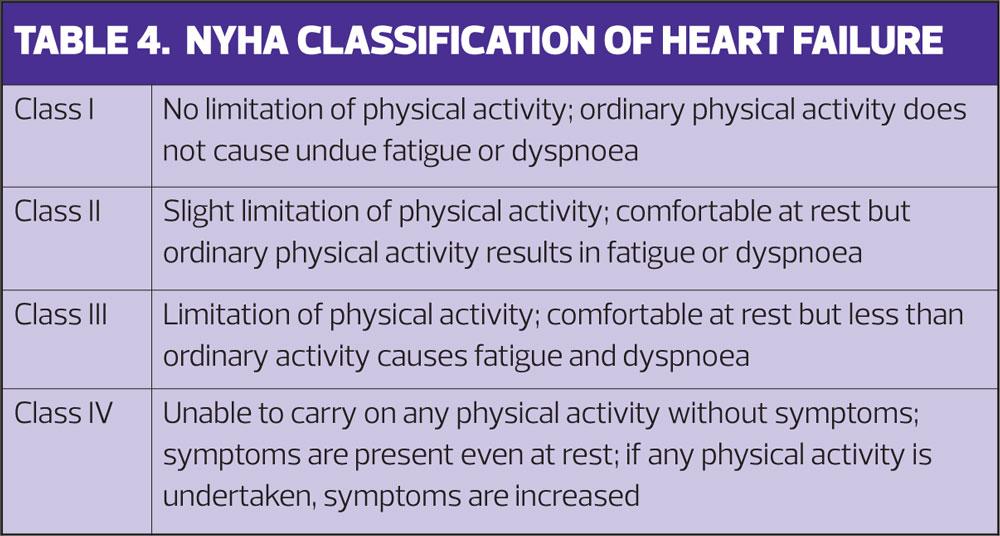
Patients with heart failure need to be regularly monitored and NICE recommends a minimum review period of six months when stable.6 In addition to routine observations, blood tests and review of medications there also needs to be a review of functional capacity. The NYHA Functional Classification,13 is the most commonly used tool and can be added to your clinical template for ease of use and to enable coding to the patient record. (Table 4) Regular use of the NYHA classification will not only help patients describe how their symptoms are affecting their lives but also enable comparison of functional capacity over time, help monitor the effects of treatments and detect deterioration.
Heart Failure and Atrial Fibrillation
Routine monitoring must include an assessment of pulse rhythm. Pulse checks, followed up by 12 lead ECG if irregular, should always be performed in patients with heart failure as they are at very high risk of concomitant atrial fibrillation (AF).8 Where AF is evident the patient should be protected from stroke by anticoagulation, unless contraindicated.8
QUALITY OF LIFE
Improving quality of life (QOL) should be a key goal of heart failure care. The impact of heart failure is likely to have a detrimental effect on the patient’s QOL and this will increase as the disease progresses.12 Similar effects are seen from all types of heart failure and studies show a direct correlation between worsening NYHA classification and decreasing QOL.14 There are tools available to measure QOL, such as the Minnesota Living with Heart Failure Questionnaire,15 and it is recommended that measurement of QOL is routinely undertaken and recorded.6,8
Depression and anxiety are common in patients with heart failure and are associated with poor QOL and poor adherence to treatment. The detection and management of these is therefore extremely important.16
As heart failure progresses and patients move towards the end of their life, the priorities of care will shift further towards QOL, rather than extension of life, and supportive palliative care should be available to all patients.
SUMMARY
Advances in early diagnosis and evidence-based treatments for heart failure have resulted in some improvements in outcomes for patients. There are, however, challenges still to be met. Significant investment has been made in many areas across the country but access to services is varied and inconsistent. As the responsibility for the diagnosis and management of heart failure becomes increasingly embedded in primary care it is essential that nursing teams are supported and enabled to fulfil their role in the care of patients with this potentially devastating condition.
ACTIVITY 1
Look at your practice heart failure register:
- What is the prevalence in your practice?
- How does this compare with the national picture?
ACTIVITY 2
Have a look at your clinical template for the review of patients with heart failure.
- Are patients with heart failure routinely asked about their symptoms and exercise tolerance?
- Is there a measure of functional capacity included such as the NYHA scale? If not, find out how this could be added
ACTIVITY 3
Do you have a heart failure specialist nurse available in your area? If so, and you don’t already know them, could you invite them to meet the practice nursing team to see how you could work together to improve patient care?
REFERENCES
1. Petersen S, Rayner M, Wolstenholme J. Coronary heart disease statistics: heart failure supplement. London: British Heart Foundation. 2002. London
2. Iyer A, Ahmed M, Fillippatos G et al. Uncontrolled hypertension and increased risk for incident heart failure in older adults with hypertension: findings from a propensity-matched prospective population study. Journal of American Society of Hypertension. 2010; 4(1): 22-31
3. England - Health and Social Care Information Centre. QOF achievement data 2015/16
4. Conrad N, Judge A, Tran J et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. The Lancet 2018; 391(10120): 572-580
5. Cowie M . The heart failure epidemic: a UK perspective. Echo Research and Practice 2017; 4: R15-R20
6. National Institute for Health and Care Excellence. Chronic heart failure: the management of chronic heart failure in adults in primary and secondary care, Clinical Guideline 108. 2010. London https://www.nice.org.uk/guidance/cg108
7. Brady L. Chronic heart failure and the supportive role of the practice nurse. Practice Nursing 2015; 26(10): 500-504
8. Ponikowski P, Voors A, Anker S et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2016 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. European Heart Journal 2016; 37 (27): 2129-2200
9. All Party Parliamentary Group on Heart Disease 2016 Focus on heart failure: 10 recommendations to improve care and transform lives, September 2016.
https://www.bhf.org.uk/-/media/files/campaigning/appg-on-heart-disease-focus-on-heart-failure-report.pdf
10. Casu G, Merella P. Diuretic Therapy in Heart Failure – current approaches. European Cardiology Review 2015; 10(1): 42–7
11. Muthumala A. Overview of devices in heart failure. E-journal of Cardiology Practice 2017; 14: no 41 https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-14/Overview-of-devices-in-advanced-heart-failure
12. Lainscak M, Blue L, Clark A, et al. Self-care management of heart failure: practical recommendations from the Patient Care Committee of the Heart Failure Association of the ESC. European Journal of Heart Failure 2011; 13:115–26
13. New York Heart Association. Criteria Committee. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th ed. 1994 Boston, MA: Lippincott Williams and Wilkins
14. Hoekstra T, Jaarsma T, van Veldhuisen D et al. (2013) Quality of life and survival in patients with heart failure. European Journal of Heart Failure 2013; 15: 94-102
15. Rector T, Kubo S, Cohn J. Patients’ self-assessment of their congestive heart failure: content, reliability and validity of a new measure, the Minnesota Living with Heart Failure questionnaire. Heart Failure 1987; 3:198 – 209
16. Toukhsati S, Driscoll A, Hare, D. Patient self-management in chronic heart failure – establishing concordance between guidelines and practice. Cardiac Failure Review 2015; 1(2): 128–31
Related articles
View all Articles