Early diagnosis of childhood cancer and why it matters
Jeanette Hawkins
Jeanette Hawkins
MSc (Adv Nursing Practice), DPSN, RSCN, RGN
Chief Nurse at the Childhood Cancer & Leukaemia Group (CCLG) and CLIC Sargent
Alison Smith
BSc Hons Clinical Practice, RM, RGN
Nurse Practitioner
Miriam Rahman
MSc Primary Care (Practice Nursing), BSc RN (Child) Paediatrics / General Practice
GPN Forum Chair. Royal College of Nursing
Practice Nurse 2021;51(6):24-28
Practice nurses are taught that childhood cancers are conditions that they will encounter infrequently in general practice – but the truth is more complex, and it is important to have a heightened index of suspicion
This article aims to raise practice nurses’ awareness of the possibility of cancer in the young, challenging some outdating ways of thinking about how rare or not childhood and young adult cancers are, and considering practice nurses’ opportunities to aid earlier diagnosis using education materials aimed at primary care.
PERSPECTIVES ON THE RARITY OF CHILDHOOD CANCER
Without exception every lecture, textbook, or presentation we have ever been exposed to about childhood cancer, states that it is rare. For those nurses who develop enhanced or advanced levels of practice and undertake comprehensive health assessments and clinical examination, we then get the mantra that ‘common things are common and rare things are rare.’ From the outset the possibility of a cancer diagnosis in the young drifts into our distant memory, taking a while to retrieve and put into everyday practice when the need arises.
Children and young people overall get distinct types of cancer different from those commonly seen in adults, so adult cancer red flag symptoms are usually less relevant. Cancer is not just one disease, there are over 200 types, of which around 80 are paediatric type, and with many sub-classifications emerging as science develops our understanding.
When broken down by cancer type it is true that some of the types of cancer seen in childhood are exceedingly rare; take hepatoblastoma, for instance, of which we see around 17 cases per year in the UK or less rare, around 480 new cases of leukaemia.1 Zoom out to look at children and young adult group as an entire population across the UK however, and we see there are around 4,300 new diagnoses per year2 resulting in a staggering 75,000 children and young people diagnosed with cancer over a 20-year period 1997-2016.1 Zoom back in, to the individual risk of a young person getting cancer, and the perspective of cancer in the young being rare changes shape again.
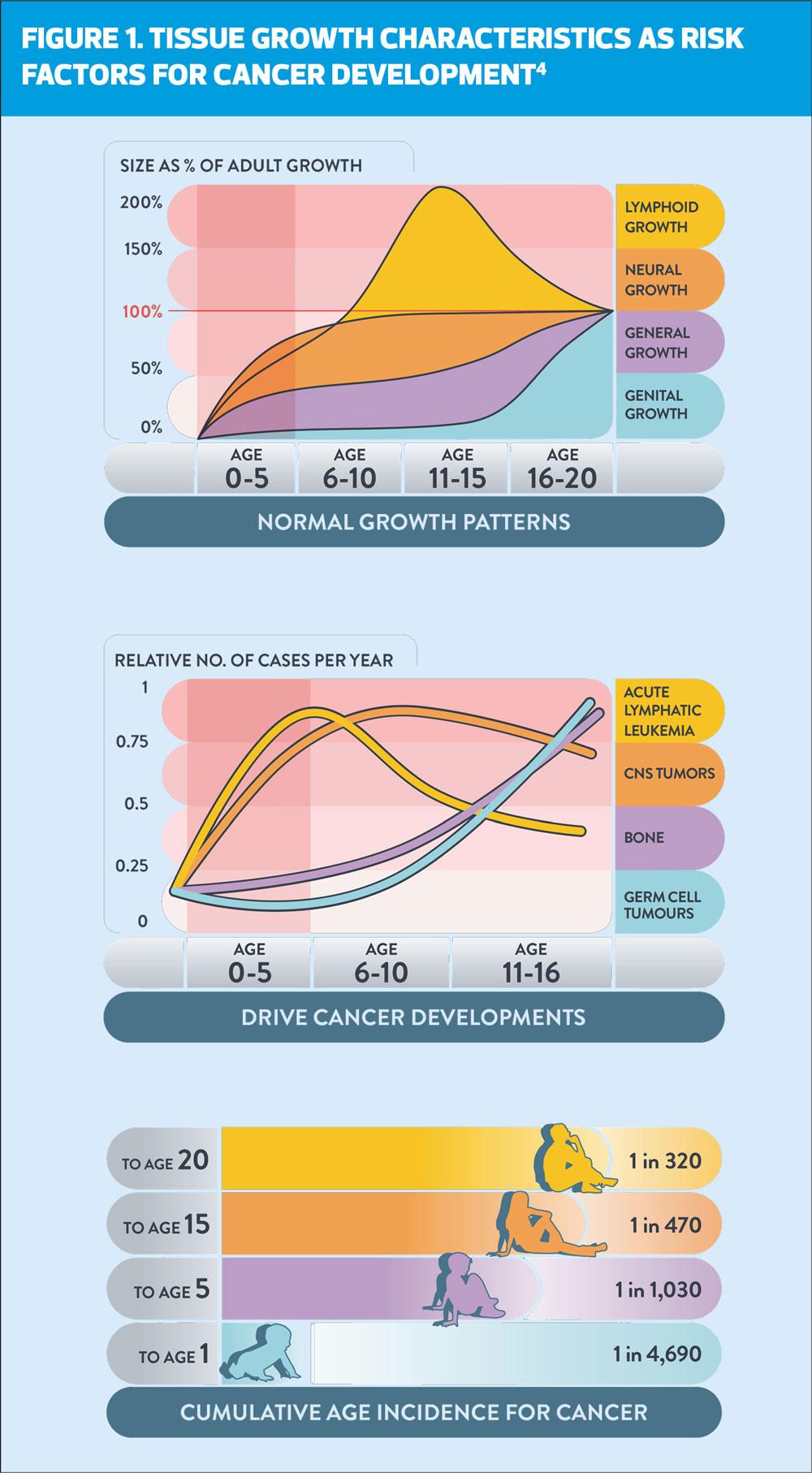
A recent review of a young person’s individual risk of cancer conducted as part of the Child Cancer Smart study3 shows that a child’s cancer risk rises from being extremely low (1 in 4700) in the first year of life, rising rapidly until 5 years of age (1 in 1000), achieving a moderate risk by 15 years (1 in 450) and a substantial risk by 20 years (1 in 320).4 ‘These risks are comparable to the risk of other common childhood conditions such as diabetes, epilepsy, and bacterial meningitis.’4
The causes and risks of childhood cancer are largely unknown,5 and it is different from the environmental, lifestyle and degenerative causes of adult cancers. Some medical conditions, such as, Down’s syndrome increase the risk of leukaemia. There is a genetic link in retinoblastoma although no genetic links identified for most other types of childhood cancer. Some childhood cancers develop from immature cells that did not develop correctly in the foetus, but which then begin to develop uncontrollably into a tumour in childhood. Other small risk groups include exposure to certain viruses (e.g., Epstein-Barr), exposure to radiation and previous cancer treatment, but for most cases there is no identifiable risk or causal factor.5,6
TRIGGERS AND DELAYS
There is an association between the development of different types of childhood cancer with physiological growth, which is demonstrated in Figure 1. As infants and children pass through normal growth patterns, we see the emergence of cancers linked to tissues which are in a state of rapid development such as bone, germ cells and neural growth, where genetic mistakes can occur.4 This goes some way to explaining why many childhood cancers develop rapidly and with acute presentation. Although conversely, other types of childhood cancer have a slower, more indolent, and silent presentation for months or even years before a diagnosis is made.7
Delays in cancer diagnoses matter across all age spectrums but focusing on children and young people there are some specific points to note. The rapid development of some cancers and particularly leukaemia, means that a child can become seriously ill in just a few days, so follow-up GP appointments for ongoing symptoms that are several weeks apart will commonly result in parents’ defaulting to A&E departments where around 53% of children are diagnosed.8 Young children may be unable to express symptoms clearly or may make examination difficult if distressed, and teens may hide or find it difficult to talk about symptoms.7,9 Sadly, cancer remains the most common cause of death in children aged 1–15 years,7 and for some tumours the survival rates in the UK are lower than in some European countries.10
Two studies, one by Smith, et al11 and one by CLIC Sargent9 found that over 50% of young people or parents with children visited their GP three times with the same symptoms before referral, and 18% to 29% respectively visited over five times before referral was made. ‘Young people we spoke to told us that they felt that their delay was avoidable because their GP initially attributed their symptoms to growing pains and adolescence.’9 Of note, waking at night with pain is a symptom of concern for childhood cancer.7,12 Late diagnosis also affects families’ trust in the healthcare system and increases families' costs if treatment is prolonged as a result.4,7,9,13
There is evidence that delays in diagnosis can result in the child presenting with more advanced disease which in turn means that, for those who survive, they may need more intense treatments over a longer duration resulting in greater morbidity, more long-term side effects and greater negative impact on their life opportunities and quality of life.
The health consequences and adverse effects of childhood cancer treatment may be physical, psychological and/or social.14 A UK study by Frobisher et al and the British Childhood Cancer Survivor Study Group risk stratified follow-up methods for survivors of childhood cancer based on their cancer type and treatment.15 Using this stratification and by analysing follow-up data they showed that 21% of the lowest level risk group (Level 1) developed further cancer or died from a non-cancer cause or were diagnosed with a treatment-related non-fatal condition by 45 years post diagnosis. This grew to 45% in the middle group (level 2) and 69% in the highest risk group (Level 3).15 Some conditions resulting from treatment do not become apparent for decades after treatment ends16 and survivors are three times more likely to die 35 years after diagnosis than those without a cancer diagnosis.17 If we can diagnose children earlier and reduce their exposure to such toxic treatments, we can reduce long-term healthcare issues that are both a trauma to the individual and burden on health and social care systems.
For some of the reasons already explained, the ‘urgent’ two-week' wait system for suspected cancer is not an appropriate pathway for children, with an exceptionally sparse number (assessed as somewhere around 1-3%) of patients being diagnosed via that route.7,18 The NICE Suspected cancer: recognition and referral guidelines19 had limitations when it came to assisting primary care practitioners to effectively identify and manage children’s cancer, until a recent supplement was published which now offers both a balanced perspective on the thresholds for suspicion against ‘unnecessary investigations, anxiety and distress.’7 However, it is recognised that because there are few ‘Red Flag’ symptoms for childhood cancer and – although not as rare as we might think, it is still not common – the recognition of childhood cancer for primary care practitioners remains a challenge.
AWARENESS RAISING
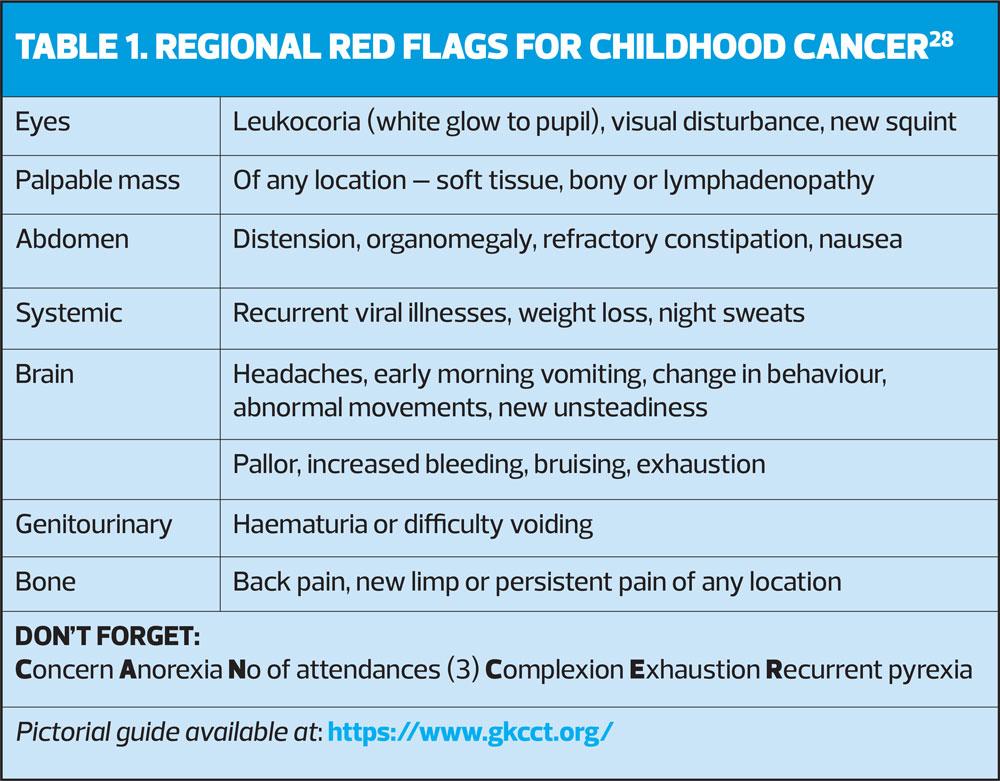
Raising awareness of specific cancers such as the NHS England ‘Be Clear on cancer: Blood in pee’ campaign20 and the Brain Tumour Charity ‘HeadSmart’ campaign have been shown to be effective21 in encouraging people to see their GP and in reducing diagnostic intervals, reducing the interval by half in the case of Headsmart.22 A similar research project and campaign called ‘Child Cancer Smart’ is now underway to try to improve resources and public and professional awareness of childhood cancer,3 including information resources for primary care. This work is currently building the evidence base through systematic reviews and meta-analysis. It is funded by the Children’s Cancer and Leukaemia Group (CCLG), Young Lives vs Cancer (CLIC Sargent), Teenage Cancer Trust, and the Grace Kelly Childhood Cancer Trust (GKCCT) who have a pictorial symptom guide summarised in Table 1, which practice nurses may find helpful. Additionally, the RAG rated symptom guides in the CCLG referral Guidance Summary make a handy quick reference chart for practitioners, available at: https://www.cclg.org.uk/publications/Information-for-GPs/Findings-that-may-be-associated-with-a-cancer-diagnosis-in-childhood/REFSUMM. There is also simplified guidance available for health visitors.23
Practice nurses have opportunities to assist in the earlier diagnosis of children’s cancers during immunisation clinics, treatment room activities, and any clinic where children may present, or even contacts in adult treatment clinics, such as smear clinics or contraception reviews, where parents may spontaneously talk about concerns about their child. Those in advanced practice roles will certainly have opportunities for staying alert to the possibility of childhood cancer.
It is recognised that Advanced Practice Nurses may come to the role from diverse backgrounds, and few will have neonatal or paediatric backgrounds. Paediatric modules on advanced practice courses tend to focus on acute paediatric emergencies, and some practice nurses may feel ill-prepared to retain lots of detailed information about various specialties. However, good general assessment skills, use of tools such as Child Assessment Forms built into electronic GP systems, the GKCCT visual guide, the NICE CYP supplement poster, trusting gut instinct, and above all ‘listening to parents’ who know their child best, will go a long way to staying vigilant to the possibility of childhood cancer.
Within the practice there will always be systems to discuss concerns with colleagues, the duty GP, or the community paediatrician.24 Some regions have developed specific pathways for assisting general practice with referral decisions for suspected cancer in childhood. The Paediatric Oncology Team at the University Hospital North Midlands is a secondary care provider taking referrals from primary care for suspected childhood cancer to investigate prior to or in conjunction with the tertiary cancer centre. The team noted a doubling of suspected cancer referrals for children following the publication of the NICE Suspected Cancer [NG12] Guidelines in 2015.19 A review of two-week-wait referrals showed that only one in 500 was cancer. They noted that GPs wanted someone to talk cases through with, as they were worried about reassuring families and were making referrals out of caution. The North Midlands team devised a telephone triage system that was fast and efficient to support referral decision-making. GPs have two routes to speak to a paediatric consultant with oncology expertise, either via switchboard to the on-rota consultant or via the Consultant Connect App, https://www.consultantconnect.org.uk/wp-content/uploads/2019/08/GP-App-start-up-guide-one-page.pdf .25 Cases were managed via same day review, oncology clinic review within 2 weeks, divert to dermatology two-week-wait referral, refer to different speciality or advice for GP monitoring.
The team also noted that many adolescent girls with breast buds were referred under the two-week-wait system. There is now an option for breast lumps to be taken off the two-week-wait pathway and moved to a one-stop adolescent breast lump clinic within 4 weeks. This evaluates well and demonstrates that a cancer referral was not necessary for pre-pubertal breast buds and caused undue distress, while offering a system to detect anything deviating from normal development. A summary of the agreed actions is provided to GPs using this triage system for confidence and audit purposes. Evaluation of the service noted a drop of 60% in referrals reducing unnecessary family distress, no confirmed cases of cancer were missed or delayed, improved time to correct management and support for GP education.26
Other regions have modified the paediatric two-week-wait referral forms to enable clinicians to contact a paediatrician discuss cases prior to referral. This ensures the urgent cases are seen immediately, less urgent cases within 2 weeks, appropriate local monitoring or investigations can be triggered or diversion to other paediatric specialties recommended. This may occur when the presenting symptoms have a more likely alternative cause such as blood in stools to gastroenterology (which is a red flag for adult cancer but much less so in paediatrics).
CONCLUSION
In summary, the aim of greater awareness and earlier diagnosis is two-fold; firstly, and more obviously to improve survival rates, and secondly to facilitate treatment at an earlier stage which may be less toxic than treatments for advanced stages of cancer.27 This reduces treatment duration for children and their families, enabling a faster return to education, careers, employment, and more normal lifestyles, and reduces the likelihood of the long-term consequences of treatment.
INVITATION The Child Cancer Smart project would like to invite healthcare professionals who work in primary care, emergency medicine and paediatrics to take part in a childhood cancer awareness survey, at https://nottingham.onlinesurveys.ac.uk/ccs_professional. We are working to improve awareness of the signs and symptoms of childhood cancer amongst both professionals and the public, and this survey is an important part of our workplan, to help us understand the current level of knowledge and identify any important gaps. We would also be grateful if you can forward to invitation to relevant colleagues who are eligible to complete the survey. You can also find out more about the Child Cancer Smart project at www.cclg.org.uk/childcancersmart. Child Cancer Smart is a project of Children’s Cancer and Leukaemia Group (CCLG) and the University of Nottingham, in partnership with Young Lives vs Cancer (formerly CLIC Sargent), The Grace Kelly Childhood Cancer Trust, and Teenagers and Young Adults with Cancer (TYAC).
REFERENCES
1. Public Health England. Children, teenagers, and young adults cancer statistics report; 2021 http://www.ncin.org.uk/cancer_type_and_topic_specific_work/cancer_type_specific_work/cancer_in_children_teenagers_and_young_adults/
2. Cancer Research UK (CRUK). Children’s Cancer Statistics; 2017 https://www.cancerresearchuk.org/health-professional/cancer-statistics/childrens-cancers and Young People's Cancer Statistics https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/young-peoples-cancers
3. Children’s Cancer and Leukaemia Group (CCLG). Child Cancer Smart; 2019 https://www.cclg.org.uk/ChildCancerSmart
4. Walker DA. Helping GPs to diagnose children’s cancer. Br J Gen Pract 2021;71(705):151-152
5. Cancer Research UK. Risks and causes of childhood cancer; 2020
https://www.cancerresearchuk.org/about-cancer/childrens-cancer/risks-causes
6. World Health Organization (WHO). Childhood cancer; 2021 https://www.who.int/news-room/fact-sheets/detail/cancer-in-children
7. Children’s Cancer and Leukaemia Group. Referral guidance for suspected cancer in children and young people; 2021. http://www.cclg.org.uk/guidelines
8. National Cancer Intelligence Network. Routes to Diagnosis Workbook 2006-2013 Version 2.0; 2015
https://data.healthdatainsight.org.uk/apps/routes_to_diagnosis/route_emergency_ctya/
9. CLIC Sargent. The best chance from the start: Improving support to identify cancer in children and young people; 2015 https://www.clicsargent.org.uk/join-our-fight/get-campaigning/our-research/
10. Craft AW, Pritchard-Jones K. UK childhood cancer survival falling behind rest of EU? The Lancet Oncology 2007;8(8):662-663
11. Smith S, Davies S, Wright D, Chapman C. The experiences of teenagers and young adults with cancer— results of 2004 conference survey. European J Oncol Nurs 2007;11(4):362-368
12. Cancer Research UK. Signs and symptoms of cancer in children; 2021 https://www.cancerresearchuk.org/about-cancer/childrens-cancer/symptoms
13. Dommett RM, Pring H, Cargill J, et al. Achieving a timely diagnosis for teenagers and young adults with cancer: the ACE (Adverse Childhood Events) “too young to get cancer?” study. BMC Cancer 2019;19;616 https://doi.org/10.1186/s12885-019-5776-0
14. Gibson F, Soanes L. 2001. Long-term follow-up following childhood cancer: Maximising the contribution from nursing. European journal of cancer Paediatric Update 2001;37(15):1859-1866
15. Frobisher C, et al and the British Childhood Cancer Survivor Study Group (BCCSSG). Risk stratification of childhood cancer survivors necessary for evidence-based clinical long-term follow-up Brit J Cancer 2017;117:1723–1731
16. Hudson MM, Ness KK, Gurney JG, et al. Clinical Ascertainment of Health Outcomes Among Adults Treated for Childhood Cancer. JAMA 2013;309(22):2371–2381.
17. Ruelen RC, Winter DL, Frobisher C, et al. British Childhood Cancer Survivor Study Steering Group. Long-term cause-specific mortality among survivors of childhood cancer. JAMA 2010;304(2):172-9
18. Galloway P, Arumugam J, Hargreaves J, et al. How effective is the 2-week urgent referral pathway in identifying paediatric cancer? Experience from a general paediatric hospital and literature review Arch Dis Child 2016;101:A8-A9. https://adc.bmj.com/content/101/Suppl_1/A8.2
19. NICE NG12. Suspected Cancer: Recognition and referral; 2015 https://www.nice.org.uk/guidance/ng12
20. National Disease Registration Service. Be Clear on Cancer: Blood in Pee campaigns – an overview of the final evaluation; 2021. https://www.ndrs.nhs.uk/be-clear-on-cancer-blood-in-pee-campaigns-an-overview-of-the-final-evaluation/
21. The Brain Tumour Charity. Impact of the HeadSmart Early Diagnosis Campaign; 2016 https://www.thebraintumourcharity.org/brain-tumour-diagnosis-treatment/child-brain-tumour-research/impact-of-the-headsmart-early-diagnosis-campaign/
22. The Brain Tumour Charity. Clinical Guideline; 2021 https://www.headsmart.org.uk/clinical/clinical-guideline/ Accessed 24th May 2021.
23. Institute of Health Visiting. Good Practice Points-Childhood Cancer: spotting the signs and symptoms; 2017 https://ihv.org.uk/for-health-visitors/resources-for-members/resource/good-practice-points/generic-gpps/childhood-cancer-spotting-signs-symptoms/
24. McInnes S, Peters K, Bonney A, Halcomb E. A qualitative study of collaboration in general practice: understanding the general practice nurse's role. J Clin Nurs (Australia) 2017;26:1960-1968. https://doi-org.rcn.idm.oclc.org/10.1111/jocn.13598
25. Consultant Connect. https://www.consultantconnect.org.uk/
26. Thompson S, Roe L, Kumar A. Two-week-wait referrals – telephone Triage Poster and Oral Presentation [OP2] at 16th International Paediatric Haematology Oncology Update Meeting (IPHOUM); 2018 http://www.iphoum.com/AbstractView.asp
27. Parker K, Hall MA. 2017, Childhood Cancers: Diagnostic Considerations. Journal for Nurse Practitioners 2017;13(8):e401-e402 DOI: https://doi.org/10.1016/j.nurpra.2017.05.017
28. Grace Kelly Childhood Cancer Trust. Regional red flags of childhood cancer card for professionals; 2021. https://www.gkcct.org/publications-professionals
Related articles
View all Articles