NICE asthma guideline: Costs, controversies, commendations and caveats
MANDY GALLOWAY
MANDY GALLOWAY
Editor, Practice Nurse
After months of delays, the single NICE guideline on asthma has been published – and has set the cat among the pigeons in the respiratory community
The long-anticipated NICE guideline on the diagnosis, monitoring and management of chronic asthma was released last month, one month after its original publication date.
The first draft guideline was widely criticised for its recommendations on objective tests to confirm a diagnosis of asthma. It was held up for several months while NICE carried out feasibility studies to establish whether it was practical to introduce spirometry and FeNO (fractional exhaled nitric oxide) into routine practice. A new publication date was also missed while NICE had talks with NHS England about implementation.
Launching the guideline, NICE said it would now recommend a phased introduction of objective testing for most people with suspected asthma.
‘This is a significant enhancement to current practice, which will take the NHS some time to implement, with additional infrastructure and training needed in primary care,’ the guideline states. ‘New models of care, being developed locally, could offer the opportunity to implement these recommendations. This may involve establishing diagnostic hubs to make testing efficient and affordable. They will be able to draw on the positive experience of NICE’s primary care pilot sites, which trialled the use of FeNO.
‘The investment and training required to implement the new guidance will take time. In the meantime, primary care services should implement what they can of the new guidelines, using currently available approaches to diagnosis until the infrastructure for objective testing is in place.
‘There is currently no gold standard test available to diagnose asthma; diagnosis is principally based on a thorough history taken by an experienced clinician. Studies of adults diagnosed with asthma suggest that up to 30% do not have clear evidence of asthma. Some may have had asthma in the past, but it is likely that many have been given an incorrect diagnosis. Conversely, other studies suggest that asthma may be underdiagnosed in some cases.
‘The diagnosis recommendations will improve patient outcomes and will be cost-effective to the NHS in the long-term; NICE’s cost impact assessment projects a saving of approximately £12 million per year in England, before implementation costs.’
In arriving at this figure, NICE made the assumption that ‘no objective testing takes place in current practice for the diagnosis of asthma in primary care’.
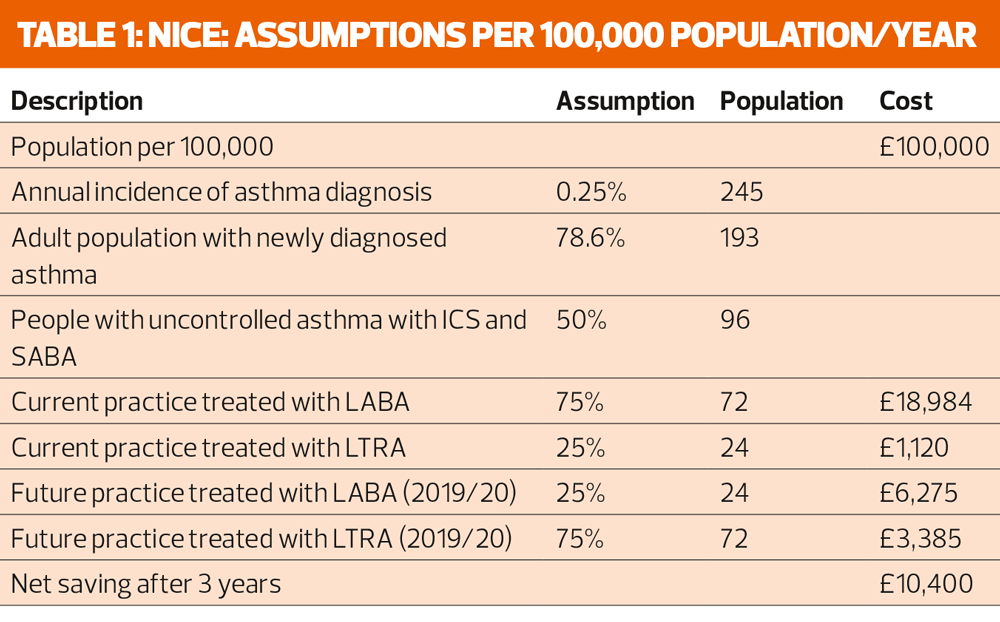
A resource impact report suggests that it will take 20 minutes of general practice nurse time to perform each objective test, at an estimated cost of £36 per hour. The nursing costs per 100,000 population have been estimated to be £9,517 a year.
In order to implement the new diagnostic pathway, practices will need to buy new FeNO machines, ranging in cost from just over £1,700 to more than £3,000 – with consumable costs on top of that. But NICE expects the number of people diagnosed with asthma to be reduced from 237 per 100,000 to 153, generating savings of £7,261.
If every practice in England were to buy its own FeNO machine, it would take 6 years to recoup the costs. But if CCGs or other groups set up diagnostic hubs, with two machines per 100,000 population, the payback period would be reduced to around 10 months. The latter scenario does not make any allowance for potential delays in diagnosis if patients had to be referred to a hub rather than be diagnosed in their GP practice.
The other factor contributing to NICE’s anticipated £12m a year saving is the recommendation to put more people whose asthma is uncontrolled with a combination of inhaled corticosteroids (ICS) and short acting beta agonist (SABA) on to a leukotriene receptor antagonist (LTRA) rather than a long acting bronchodilator (LABA), as recommended by the BTS/SIGN British guideline for the management of asthma.
The annual cost of treatment with a LABA is £319, and with an LTRA is £48, so making this switch for people with newly diagnosed asthma which is not well controlled on low dose ICS plus SABA would save £10,400 per 100,000 population after 3 years, and, for the whole population of England, savings would rise to around £5.7 million from 2019-20 onwards.
NICE says that putting its recommendations into practice ‘can take time’ but changes that can be done quickly – like changes in prescribing practice – should be shared quickly. ‘This is because healthcare professionals should use guidelines to guide their work – as is required by professional regulating bodies such as the General Medical and Nursing and Midwifery Councils.
‘Changes should be implemented as soon as possible, unless there is a good reason for not doing so (for example, it would be better value for money if a package of recommendations were all implemented at once).’
PCRS response to the NICE guideline
Ever since NICE made known the intention to produce its own asthma guideline, PCRS-UK has argued strongly for a single consistent comprehensive and regularly updated asthma guideline for the four nations of the UK. PCRS-UK is concerned that the NICE guideline will create uncertainty for primary care clinicians, because in some areas they recommend a different approach to the BTS/SIGN (British Thoracic Society and Scottish Intercollegiate Guidelines Network) guideline, which has set the evidence based standard for the UK since 2003. We do however recognise the importance of NICE’s incorporation of cost-effectiveness analysis, which is not addressed by BTS/SIGN.
PCRS-UK believes there is a risk that that multiple guidelines will confuse clinicians about the most appropriate approach to asthma diagnosis and management and notes that there are important areas of asthma care – such as the management of acute severe asthma – which are not covered by the NICE guideline.
Until the problem of multiple UK guidelines is rectified, PCRS-UK will have an important role to play in helping primary care professionals decide on the best course of action in the face of differing advice.
The two main areas where the NICE guideline recommends a different approach to established practice are:
- The role and sequencing of objective tests in asthma diagnosis, including FeNO testing (fractional exhaled nitric oxide), spirometry and peak flow measurement
- The use of leukotriene receptor antagonists (LTRAs)
THE ROLE OF OBJECTIVE TESTS
NICE advocates a complex algorithm involving both spirometry and FeNO testing in primary care with peak flow testing only in cases of doubt. Trials of treatment are discouraged except in patients who present when acutely unwell.
We have argued for the routine initial use of peak flow monitoring as an objective test. This test is easily available in primary care and easy to repeat, whether or not the patient’s symptoms warrant a trial of treatment
We have significant concerns about an approach that recommends greater reliance on objective testing at a single point in time. This risks not detecting asthma if the patient is asymptomatic at the time of testing. The majority of people with asthma will have normal spirometry when it is tested; these false negatives mean it is not possible to rule out asthma with spirometry. There are also problems with prompt access to quality assured diagnostic spirometry in primary care.
There are both false positives and false negatives with FeNO testing, and it may not detect asthma in a patient with a chest infection (both FeNO testing and spirometry testing should be deferred in this situation) or in patients who smoke.
We see a role for FeNO testing, where the structured clinical assessment suggests an intermediate probability, in line with BTS/SIGN guidance. This acknowledges that a positive FeNO test indicates the presence of eosinophilic inflammation and increases the probability of asthma.
We are concerned that NICE’s recommendation to use FeNO testing in all people with suspected asthma as a primary investigation raises major implementation challenges and could have a number of unintended consequences. FeNO testing is not widely available in primary care. It carries significant cost – both in terms of initial investment and the ongoing cost of consumables. It is therefore unlikely to be a viable option for all practices, but may be more realistically provided as part of a locality based diagnostic service.
A perceived mandatory requirement for FeNO and spirometry testing may increase referrals into secondary care. This risks deskilling primary care, and overloading secondary care services. NICE did not include in its analysis any costing for increased referrals to secondary care for asthma diagnosis. It is very important that primary care should have rapid access to specialist services for patients in whom the diagnosis is in doubt.
We are pleased to see that NICE, in the final guideline published today (29 November 2017), recommends a ‘phased implementation’. However, this does not address the more fundamental concerns we have about the weaknesses of testing at a single point in time. Balanced against other priorities within respiratory care and more broadly in the NHS, we do not see the widespread implementation of FENO testing as high priority for localities to address.
PCRS-UK recommendations:
- We recognise the value of objective tests, but would point out their limitations when undertaken at a single point in time.
- We recommend peak flow monitoring as the initial objective test.
- We see a role for FeNO testing, where the structured clinical assessment suggests an intermediate probability, in line with BTS/SIGN guidance.
MANAGEMENT OF ASTHMA
The recommendations in this guideline are broadly consistent with BTS/SIGN except in one respect – the use of leukotriene receptor antagonists (LTRAs).
NICE and SIGN/BTS have different advice on the choice of first-line add-on treatment to low dose inhaled corticosteroids (ICS). Clinicians have a choice between adding an LTRA or adding a long acting beta agonist (LABA) for patients not controlled on low dose ICS. Whereas BTS/SIGN recommends LABA as first line add-on to ICS, NICE has undertaken health economic analyses which factor in the lower cost of LTRA, and have determined that though LABA and LTRA have broadly equivalent clinical efficacy, the more cost effective option is LTRA.
While clinicians currently add in a LABA as first choice to ICS at this stage, in line with BTS/SIGN recommendations, the evidence review undertaken by NICE suggests that there is little to choose between LABA and LTRA. LABA are considered marginally more effective than LTRA in controlling exacerbations. In addition, they are given as combination inhalers so that non-adherence with ICS is prevented. However LTRA are substantially cheaper than LABA and cost is a key consideration for the NHS.
PCRS-UK recommendations
- It is NOT appropriate to switch a patient whose symptoms are well controlled on current treatment, so there is no need to change the medication of patients who are well controlled on LABA/ICS.
- Unless there is good reason to the contrary, try LTRA as first line add on therapy to ICS in adults.
- PCRS-UK supports the use of a paediatric low dose ICS with LTRA, as first line add on treatment in children with asthma. [text] If this combination is ineffective, switch the LTRA for a LABA.
Dr Duncan Keeley, PCRS-UK Policy Lead says: ‘NICE is an excellent organisation which plays a vital role in improving the cost effectiveness of our health services. But PCRS UK has always questioned the wisdom of seeking to replace the widely respected BTS/SIGN guideline for asthma - which was itself approved by NICE.
‘We feel it would be better for NICE to contribute its expertise in cost benefit analysis to the options in the existing comprehensive BTS/SIGN guideline rather than seeking to replace it. There is a strong preference in the primary care community for a single comprehensive asthma guideline for the four nations of the UK.
‘We are concerned at the potential for confusion among health professionals caused by conflicting guideline advice. We identified major potential difficulties in implementation of the recommendations in the NICE Asthma: diagnosis, monitoring and chronic asthma management guideline - views shared by many other organisations in the respiratory field - and we advised against its publication. We would encourage NICE and BTS/SIGN to cooperate on a single guideline in future. We will provide advice in the meanwhile to the primary care community about how to reconcile the conflicting guideline advice that we now have.’
The guideline in a nutshell
CLINICAL HISTORY
Take a structured clinical history in people with suspected asthma. Specifically check for:
- Wheeze, cough or breathlessness, and any daily or seasonal variation in these symptoms
- Any triggers that make symptoms worse
- A personal or family history of atopic disorders
Do not use symptoms alone without an objective test to diagnose asthma
Examination
Examine people with suspected asthma to identify expiratory polyphonic wheeze and signs of other causes of respiratory symptoms. Be aware that even if the examination results are normal, the person may still have asthma.
Initial treatment and objective tests
Treat people immediately if they are acutely unwell at presentation, and perform objective tests (FeNO, spirometry and peak flow variability) if the equipment is available and testing will not compromise treatment of the acute episode.
If objective tests cannot be done immediately, carry them out when acute symptoms have been controlled, and advise people to contact their healthcare professional immediately if they become unwell while waiting for objective tests.
Empiric treatment with ICS may affect the results of spirometry and FeNO.
Skin prick tests, serum total and specific IgE, peripheral blood eosinophil count and exercise challenge are not recommended.
Occupational asthma
Check for possible occupational asthma by asking employed people with suspected new-onset asthma or asthma that is poorly controlled if their symptoms are better on days away from work or on holiday.
Record answers for later review, and refer people with suspected occupational asthma to an occupational asthma specialist.
Diagnosing asthma in young children
For children under 5 with suspected asthma, treat symptoms based on observation and clinical judgement. If they still have symptoms when they reach 5 years, carry out objective tests.
If the child is unable to perform the tests, try again every 6 to 12 months until satisfactory results are obtained.
OBJECTIVE TESTS
CCGs should consider establishing asthma diagnostic hubs to achieve economies of scale and improve the practicality of implementing the recommendations.
FeNO
Offer a FeNO test to adults, aged 17 and over, if a diagnosis of asthma is being considered. A level of 40 parts per billion (ppb) or more is positive.
Consider a FeNO test for children and young people (aged 5 to 16) if there is diagnostic uncertainty after initial assessment and they have either:
- Normal spirometry, or
- Obstructive spirometry with a negative bronchodilator reversibility (BDR) test
Regard a FeNO level of 35ppb or more as a positive test.
Spirometry
Offer spirometry to adults, young people and children aged 5 and over if a diagnosis of asthma is being considered. Regard a forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio of less than 70% as positive for obstructive spirometry.
Bronchodilator reversibility
Offer a BDR test to adults (aged 17 and over) with an FEV1/FVC ratio <70%. An improvement in FEV1 of 12% or more, together with an increase in volume of 200ml or more, as positive.
Consider offering a BDR test to children and young people (aged 5 to 16) with an FEV1/FVC ratio <70%. Regard an improvement in FEV1 of >12% as positive.
Peak flow expiratory flow variability
Monitor peak flow variability for 2–4 weeks if there is diagnostic uncertainty after initial assessment and a FeNO test – regard a value of more than 20% variability as positive:
- In adults (aged 17 and over) who have:
Normal spirometry or
Obstructive spirometry, positive BDR but FeNO level of 39ppb or less.
- In children and young people (aged 5 to 16) who have:
Normal spirometry or
Obstructive spirometry, negative BDR and a FeNO level of 35ppb or more
PRINCIPLES OF PHARMACOLOGICAL TREATMENT
Consider possible reasons for uncontrolled asthma before starting or adjusting treatment, including:
- Alternative diagnoses
- Lack of adherence
- Suboptimal inhaler technique
- Smoking (active or passive)
- Occupational exposures
- Psychosocial factors
- Seasonal or environmental factors
Review response to treatment in 4–8 weeks after starting or adjusting medication for asthma.
If needed, offer daily ICS maintenance therapy.
Adjust the dose of ICS maintenance therapy over time, aiming for the lowest dose required for effective asthma control.
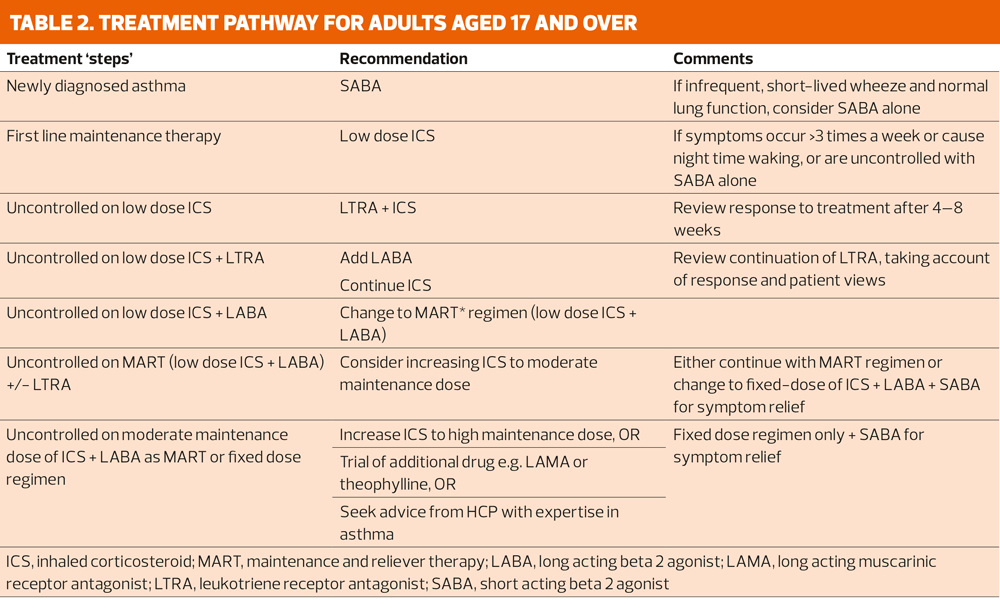
The treatment pathway for adults aged 17 and over is shown in Table 2.
TREATMENT FOR CHILDREN/YOUNG PEOPLE 5 – 16
Children and young people (aged 5 – 16) should be offered a SABA as reliever therapy. If symptoms are infrequent and short-lived, and lung function is normal, consider SABA alone.
If symptoms at presentation (more than 3 times a week or causing waking at night, or uncontrolled on SABA alone), offer a paediatric low dose of ICS.
If asthma remains uncontrolled, consider adding LTRA in addition to ICS, and review response in 4–8 weeks. If it remains uncontrolled, consider stopping LTRA and starting LABA in combination with ICS.
If asthma is still uncontrolled, consider a MART regimen, ensuring the child or young person understands and is able to comply with this approach.
The next step if control is not achieved is to increase ICS dose to moderate paediatric maintenance dose – either continuing MART or switching to fixed dose regimen, with SABA as reliever therapy.
If still uncontrolled, seek advice from a healthcare professional with expertise in asthma and consider either:
- Increasing the ICS dose to paediatric high maintenance dose (fixed dose regimen) with SABA for symptom relief, or
- Trial of an additional drug e.g. theophylline
TREATMENT OF CHILDREN UNDER 5
It can be difficult to confirm asthma diagnosis in young children until they are able to undergo objective tests. For those with confirmed or suspected asthma:
- Offer SABA as reliever therapy
- Consider 8-week trial of paediatric moderate dose ICS for children with symptoms more than 3 times a week, or night time wakening, or suspected asthma uncontrolled with SABA alone
- After 8 weeks, stop ICS treatment and monitor child’s symptoms.
- If symptoms did not resolve during trial period, consider alternative diagnosis
- If symptoms resolved then recurred within 4 weeks of stopping ICS, restart ICS at paediatric low dose
- If symptoms resolved then recurred after 4 weeks of stopping ICS, repeat 8-week trial of ICS at paediatric moderate low dose
- If suspected asthma is uncontrolled in children under 5 on a paediatric low dose of ICS as maintenance therapy, consider adding LTRA.
- If suspected asthma is uncontrolled on paediatric low dose ICS + LTRA, stop LTRA and refer child to an HCP with expertise in asthma for further investigation and management
SELF-MANAGEMENT
Offer an asthma self-management programme – written personalised action plan and education – to adults, young people and children with a diagnosis of asthma, and their families or carers if appropriate.
Increasing ICS within self-management plan
For adults aged 17 and over, offer an increased dose of ICS for 7 days when asthma control deteriorates. Clearly outline how and when to do so, and what to do if symptoms do not improve.
When increasing ICS dose:
- Consider quadrupling the regular ICS dose
- Do not exceed maximum licensed daily dose
DECREASING MAINTENANCE THERAPY
For patients whose asthma has been controlled for at least 3 months, consider decreasing current maintenance therapy. Stop or reduce dose of medicines in an order that takes account of clinical effectiveness when introduced, side effects and the person’s preference.
Only consider stopping ICS treatment completely for people who are using low dose ICS alone as maintenance therapy, and are symptom free.
- You can see a more detailed guideline summary, including advice on monitoring and definitions of doses here
REFERENCE
NICE NG80. Asthma: diagnosis, monitoring and chronic asthma management, November 2017. https://www.nice.org.uk/guidance/ng80/
Related articles
View all Articles