Childhood asthma — a challenge for the future
Viv Marsh
Viv Marsh
RGN, RSCN, SCPHN, PGCE
Asthma and Allergy Clinical Lead, Education for Health
Steve Holmes
MMedSci, MBChB, FRCGP, DRCOG
Education Lead, Primary Care Respiratory Society; General Practitioner, The Park Medical Practice, Shepton Mallet
For many clinicians the diagnosis and management of asthma in children feels uncertain and challenging. This article aims to provide practical guidance with a sound evidence base
Asthma remains a serious, potentially life threatening condition, yet the recent National Review of Asthma Deaths (NRAD),1 highlighted widespread ‘complacency’ among patients, families and health care professionals (HCP). The review has challenged HCPs across all areas to improve care. The tragic deaths of children (many of which are preventable) are events that should not happen in the modern NHS.1
PREVALENCE AND IMPACT
Asthma is one of the most common long-term conditions, and can affect people for a large percentage of their life. It is common in children and young people, and the younger the person is, the more challenging the diagnosis. Indeed, many specialists suggest that a diagnosis of asthma should rarely, if ever, be made in children under 5 years of age.2,3
It is difficult to be sure of the exact prevalence of asthma in UK. The widely quoted figures from Asthma UK suggest a prevalence of 5.4 million people, of whom 1.1 million are thought to be children.4 However, the evidence base for this statistic is very unclear. Quality and Outcomes Framework (QOF) data (which does not collect asthma data in children under the age of 8 years) across the United Kingdom suggest the prevalence in adults is 3.85 million,5 but there is no ready breakdown for children.
It is likely that the true prevalence is lower than 3.85 million. There appears to be good evidence that prevalence figures have been reducing over time. This may be, in part, due to the diagnostic labels used.6 NICE, referring to some Canadian research, has recently suggested that 30% of people are overdiagnosed.7 Even these lower figures may be overestimates.
There is no doubt, however, that asthma is common and it is largely managed in primary care by practice nurses. There is also no doubt that, for young people, its impact on quality of life, school attendance, sport and other activity can be significant. It can result in significantly lower self-esteem,8 and the health care burden is high.9
Although symptoms can resolve, asthma is, for many, a lifelong condition. Management therefore focuses on gaining and maintaining optimal control and minimising its impact.10
CAUSE AND TRIGGERS
The cause of asthma is not fully understood but is known to be multi-factorial,10 and a wide range of triggers have been identified. (Box 1) There is, however, strong evidence that tobacco smoking by the mother during pregnancy and exposure to tobacco smoke in the early years increases the risk of asthma.11
DIAGNOSIS
Accurate diagnosis is essential. Only a confident, accurate diagnosis allows appropriate choice of evidence-based treatments. Without this, parents, children and HCPs are left with uncertainty, about the future and about the value of prescribed treatments.
In both adults and children there are four typical symptoms; wheeze, shortness of breath, chest tightness and cough – though cough alone is a rare presenting feature of asthma in any age group.3 A diagnosis of asthma requires the presence of more than one of these typical symptoms.
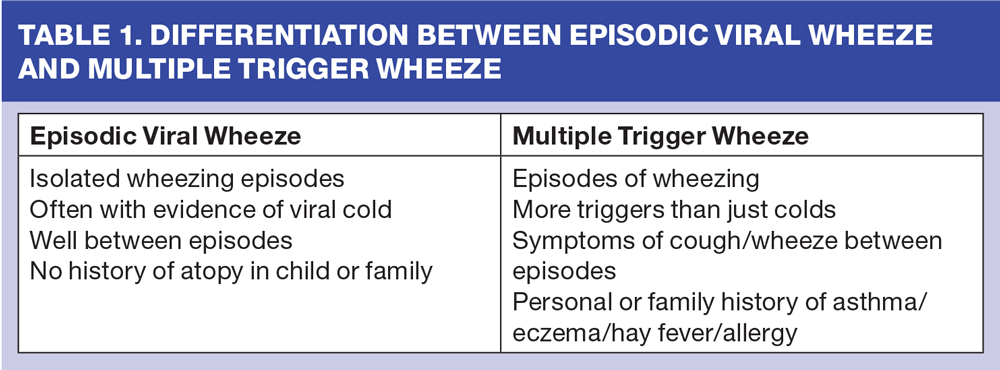
Children under 5 years (Episodic viral wheeze or multiple trigger wheeze)
European guidance for pre-school children has a good evidence base and suggests that we should avoid a diagnosis under the age of 5 years.3,12 It suggests dividing wheezy children in this age group into:
- Episodic viral wheezers, who are well between times and only have symptoms precipitated by viral upper respiratory tract infections
- Multiple trigger wheezers, where the differentiation of when a child wheezes is more blurred. (Table 1)
Children over 5 years
The British Guideline on the Management of Asthma recommends that the diagnosis in children should be based on identification of characteristic signs and symptoms where there is no alternative explanation for them. Sound clinical judgement, based on a thorough history and examination, will identify the probability of asthma. Categorising the probability as low, intermediate or high enables selection of the appropriate next steps.10
History taking should focus on symptoms, previous medical history, family medical history, birth history and environmental factors. (Box 2) Examination should focus on the chest, child’s growth and typical atopic features. (Box 3) The link between atopy and asthma is soundly proven and when there is evidence of atopy, either in the child or an immediate family member, the likelihood of asthma is greatly increased.
Low probability of asthma
Features that lower the probability of asthma include symptoms that occur only in relation to viral infections/colds, wet cough, failure to thrive and dizziness/peripheral tingling.
While asthma is common other causes of the symptoms must be excluded. Differential diagnoses include:
- Normal childhood illness
- Episodic viral wheeze
- Cystic Fibrosis/bronchiectasis
- Chronic Lung Disease of Prematurity
- Cardiac disease.
Respiratory infections and viral illness are very common in children, often resulting in frequent episodes of wheeze or cough that lasts for 3 weeks or more. Young children, particularly those of pre-school age, can expect to contract at least 10 viral infections per year. To parents it may seem that their child is never well and always has a cough. Careful questioning, to determine if there are any symptom free periods, is important and symptom diaries may be helpful.13
Where the probability of asthma is low alternative diagnoses and, if necessary, therapeutic interventions should be sought.
High probability of asthma
Where the probability of asthma is high a trial of treatment based on current severity should be commenced and response to treatment assessed after 6-12 weeks. If a response is absent or poor:
- Review the clinical history and examination
- Check the child’s ability to use the medication (inhaler technique)
- Assess adherence.
It is unusual for asthma not to respond to treatment. If there is no obvious alternative diagnosis and inhaler technique and adherence are good, it would be appropriate to refer for a specialist opinion.
Intermediate probability of asthma
When there is not enough evidence to confirm or refute a diagnosis of asthma the probability is recorded as intermediate and one of the following options selected:
- Watchful waiting with review
- Trial of treatment with review (and then stopping treatment for a period to see if symptoms recur)
- Objective measures of lung function.
If symptoms have resolved it may be difficult to determine if this was a response to treatment or a natural resolution. If there is any doubt, a carefully monitored trial of withdrawing treatment may be helpful. Symptom diaries and home peak flow monitoring can facilitate monitoring.
Diagnostic testing
There is no definitive test to confirm the diagnosis of asthma but it is recommended that objective measures of lung function are used to demonstrate reversibility or variability.
Spirometry (pre and post bronchodilator) is the preferred objective measure, though recent draft guidance from NICE is considering spirometry and forced exhaled nitric oxide measurement. Quality assured diagnostic spirometry is increasingly available in primary care and it is possible for children from about 5 years of age to perform the test. In practice, however, attempts at spirometry are often not made until children are older.
Peak Expiratory Flow rate (PEF) measurements can also be used. PEF measurement is a simple procedure, widely available in primary care and can be used by children from about 6 years of age. An increase of 12% or more in FEV1 or PEF 15 minutes post bronchodilator indicates reversible airflow obstruction, supporting a diagnosis of asthma. However, as asthma is also characterised by variability, airflow obstruction may not be present at the time of testing. Isolated measurements and tests may therefore not be helpful and negative tests cannot exclude the diagnosis.
Serial PEF, carried out morning and evening for 2 weeks, can be used to demonstrate diurnal variability, although this is not currently recommended as first line in the British guidance. Variability of 20% or more on at least 3 days per week supports the diagnosis of asthma. There are, however, a number of limitations to this method and results should be interpreted cautiously and with regard to the clinical history.
Parents and children should be encouraged to become actively involved in diagnosis and management. It is extremely helpful to openly discuss with them why you think the diagnosis may or may not be asthma. This will often help them understand the dilemma and uncertainty. If you think asthma likely then you can point parents to reputable information sources, (See Resources) and encourage them to come back with questions and their own thoughts.
MANAGEMENT AND MONITORING
Non-pharmacological management
Many parents will want to try non-pharmacological interventions and there is a good review in the British guideline.10 In summary, it is important to discuss avoidance of exposure to tobacco smoke and obesity, and, for some people, breathing exercises may be helpful. The evidence for other non-pharmacological interventions is much less well proven.
Pharmacological management
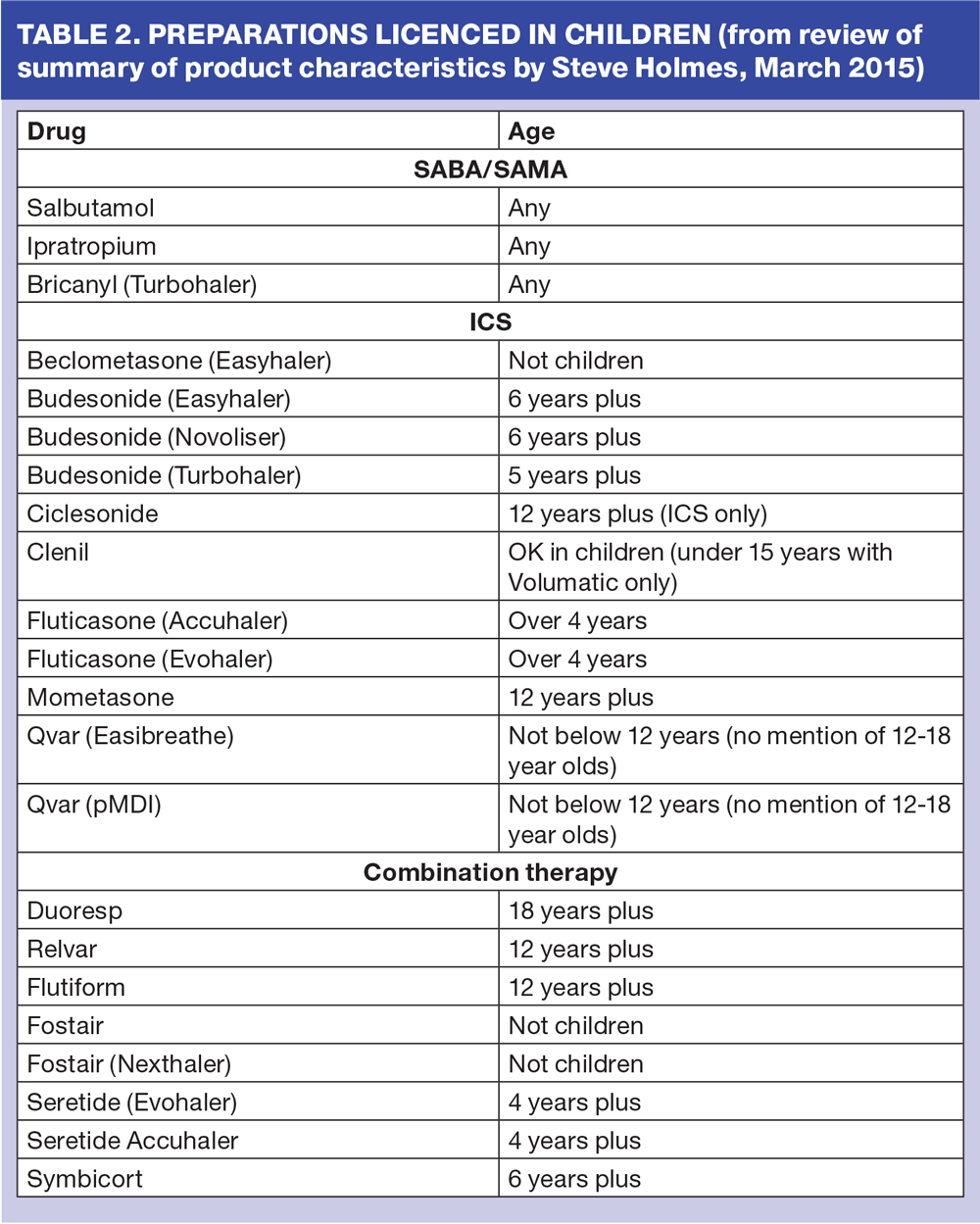
Pharmacological management is relatively straightforward as there are a limited number of drugs licensed for use in children. (Table 2) Usually, in mild intermittent asthma, it would be acceptable to provide the child with one or two short-acting beta agonist (SABA) inhalers per year, but you would not expect both to be fully used. If a child needs to use a SABA inhaler twice a week or more on a regular basis, addition of an inhaled corticosteroid (ICS) is indicated. Further step ups in treatment need to be considered if control remains poor despite good inhaler technique and adherence with ICS. Long acting bronchodilators (LABA) are licensed from 5 years of age and must always be used in combination with ICS. Anti leukotriene receptor antagonists (LTRA) are also an option at step 3 for children over 5 years of age and at step 2 under 5 years.
Many parents are concerned about ICS and their potential long term implications. People have differing health beliefs and may have been exposed to a variety of influences and ideas: for example, ICS use will mean that a child will not able to do sport, or that ICS will not work when it is needed if things worsen, are not uncommon beliefs. It is important to establish what their concerns are rather than make assumptions about what these might be.
A child with significant, ‘difficult’ asthma may fail to thrive and will lose height from the predicted value, commonly to around 15cm height loss. The Bush review14 suggests that a child commenced on treatment may lose 1.5cm from predicted height. While treatment may make your child very slightly smaller, no treatment will make them much smaller! The bottom line: there is currently no evidence of long term problems with normal dose ICS in prolonged use in children.14
Inhaler technique
Correct inhaler technique is one of the most important aspects of any review.15 The best inhaler device for any child is the one that they can, and will, use correctly.
Metered Dose Inhalers (MDI) with a spacer are recommended for ICS in all age groups.16
A spacer with a facemask can be used by younger children, usually up to approximately 4 years of age, or by anyone who is unable to use a mouthpiece effectively.
Some children prefer not to use a spacer device, increasingly as they get older, and it is important to remember that by the age of 11 more than half of children will be taking their medication without parental guidance.17 Where possible the use of a spacer should be encouraged and explanations about their effectiveness in delivering the drug to the airways, in terms the child understands, may help. Parents are also more likely to be supportive if they understand this. However, if the child chooses not to use a spacer other options to optimise drug delivery must be explored. The use of an MDI without spacer for ICS must be avoided. Dry powder inhalers and breath actuated MDIs can be useful alternatives. It is vital that the child is able to demonstrate an effective inhaler technique before being sent off with a prescription.
Treatments for episodic viral wheeze and multiple trigger wheeze
For episodic viral wheeze or multiple trigger wheeze it is acceptable, with mild symptoms, to adopt watchful waiting and not initiate drug therapy. This will often be in discussion with the parents.
Strong evidence for therapy for episodic viral wheeze in children under 5 years is lacking. The best evidence, if drug therapy is indicated, is for salbutamol at normal doses, used during the symptomatic phase, via a spacer. There is some evidence from the USA for use of montelukast during this acute phase. There is no evidence for use of inhaled or oral corticosteroids.
For multiple trigger wheeze in children under 5 years, if treatment is being considered, then it should be in line with British asthma guidelines, with low dose ICS as a cornerstone.
REVIEW
It is important to cover a number of key areas of clinical care which can be summarised in six steps. (Box 4)
When assessing whether the condition is asthma or another problem we need to assess control. Good control, as defined by the Global Initiative for Asthma (GINA), is:
- Minimal use of short acting beta agonists
- Normal lung function
- No impact on school/home activities
- No waking at night
- No requirement for treatment for exacerbations
- No admissions.18
Three courses of oral corticosteroids in a year demands specialist review. Twelve SABA inhalers in a year is a significant sign of poor control and demands urgent, careful review.
PERSONALISED ASTHMA ACTION PLANS AND THE NRAD REPORT
The latest British Asthma guideline,10 emphasises personalised asthma action plans (PAAP) as a fundamental cornerstone to asthma care, in association with regular review and patient education. There is good evidence that PAAPs reduce hospital admissions and need for acute medical help and emergency department visits, and improve quality of life and overall function.
Asthma UK produces good quality PAAPs which can be now printed from several of the GP computer programmes. The guidance suggests the following components for a PAAP:
1. Trigger for action based on peak flow or symptoms (peak flow based on percentage of best)
2. Standard written instructions (though many of our patients may have a low reading age or do not speak English). Traffic light symbols (red, amber, green) are no better than standard written plans
3. Plans should usually include two to four parameters (common ones being – when to increase use of SABA, when to start prednisolone, when and how to seek medical advise if symptoms not settling).10
The importance of PAAP was reinforced in the NRAD report.1 NRAD has been the world’s biggest review of asthma deaths. All deaths within the UK were considered and it was possible, after excluding many, to review 195 cases. Of all of those who died:
- 45% died without seeking medical assistance in their last attack
- 43% had not been seen in primary care in the last year
- Only 23% had a PAAP.
There were other key learning points:
- Adherence to ICS use was consistently poor
- A few patients were prescribed long acting beta agonists without ICS
- A high percentage (39%) had received more than 12 SABA inhalers in a year.
Key findings for children (who accounted for 28 of these cases) were a lack of awareness of signs of worsening asthma and risk of adverse outcomes.
Concise recommendations from NRAD, fundamental to primary care, are summarised in Box 5.
CONCLUSION
Asthma in children is a common and challenging condition for clinicians to manage. The diagnosis in the under-5 age group is now easier to manage using the ‘episodic viral wheeze’ or ‘multiple trigger wheeze’ labels. In the over-5s the challenge is in making a robust diagnosis based on probability, ideally supported by good evidence of reversibility and response to treatment.
There are a few worthwhile non-pharmacological interventions (smoking cessation, weight management and, possibly, breathing exercises) along with good pharmacological options. Often the challenge is to work with children and their parents so that they understand how and when to use the medications.
Finally, we are challenged to ensure that our patients do not die from asthma, that they are provided with a PAAP and that we monitor their inhaler technique, use of medication and triggers and positively work with them to improve their outcomes.
REFERENCES
1. Royal College of Physicians of London, British Thoracic Society. British Lung Foundation, Why asthma still kills: The National Review of Asthma Deaths (NRAD) Confidential Enquiry Report. London: Healthcare Quality Improvement Partnership, 2014. https://www.rcplondon.ac.uk/sites/.../why -asthm-still-kills-full-report.pdf
2. Bush A, Grigg J, Saglani S. Managing wheeze in preschool children. British Medical Journal 2014:348;g15
3. Brand PLP, Caudri D. Eber E, et al. Classification and pharmacological treatment of preschool wheezing: changes since 2008. European Respiratory Journal 2014;43(4): 1172-7
4. Asthma UK. Asthma Facts and Statistics. 2014 http://www.asthma.org.uk/asthma-facts-and-statistics
5. NHS Information Centre. Quality and Outcome Framework 2012.2013
6. Ross Anderson H, Gupta R. Strachan DP, Limb ES. 50 years of asthma: UK trends from 1955 to 2004. Thorax 2007;62(1):85-90
7. Aaron SD, Vandemheen KL, Boulet L-P, et al. Overdiagnosis of asthma in obese and nonobese adults. Canadian Medical Association Journal 2008;179(11):1121-31
8. Seigel WM, Golden NH, Gough JW, Lashley MS, Sacker IM. Depression, self-esteem and life events in adolescents with chronic diseases. Journal of Adolescent Health Care 1990;11(6):501-4
9. Wolfe I, Cass H, Thompson MJ, et al. Improving child health services in the UK: insights from Europe and their implications for NHS reforms. British Medical Journal 2011;342:d1277
10. British Thoracic Society / Scottish Intercollegiate Guideline Network. British Guideline on the Management of Asthma. Edinburgh: 2014.https://www.brit-thoracic.org.uk/guidelines-and-quality-standards/asthma-guideline/
11. Polosa R, Thomson NC. Smoking and asthma: dangerous liaisons. European Respiratory Journal 2013;41(3):716-26
12. Brand P, New guidelines on recurrent wheeze in preschool children: implications for primary care. Primary Care Respiratory Journal 2008;17(4):243-5
13. NICE. Respiratory tract infections – antibiotic prescribing; Prescribing of antibiotics for self-limiting respiratory tract infections in adults and children in primary care. 2008 National Clinical Guideline 69 https://www.nice.org.uk/guidance/cg69
14. Bush A, Inhaled corticosteroid and children’s growth. Archives of Disease in Childhood 2014;99:191-2
15. Al-Jahdali H, Ahmed A, Al-Harbi A, et al. Improper inhaler technique is associated with poor asthma control and frequent emergency department visits. Allergy, Asthma & Clinical Immunology 2013;9(1):8
16. NICE. Inhaler devices for routine treatment of chronic asthma in older children (aged 5-15 years). 2002, TA38 https://www.nice.org.uk/guidance/ta38
17. Orrell-Valente JK, Jarlsberg LG, Hill LG, Cabana MD. At what age do children start taking daily asthma medicines on their own? Pediatrics 2008;122(6):e1186-92
18. Global Initiative for Asthma. Pocket Guide for Asthma Mnagement and Prevention (for adults and children over 5 years). 2015 http://www.ginasthma.org
Related articles
View all Articles