CVD prevention: start earlier, measure smarter, treat to lower targets
Professor Ahmet Fuat MBChB PhD FRCGP FRCP FRCPE FESC FPCCS PGDiP Cardiology CertMedEd DRCOG DFFP. GPSI Cardiology, Orchard Court Surgery, Darlington, County Durham.
Practice Nurse 2025;55(5):17-21
Cardiovascular disease mortality in the UK is rising for the first time in decades. While there have been dramatic developments in CVD prevention, practice is lagging behind: six steps to improve detection, risk management and treatment
Cardiovascular disease (CVD) remains the UK’s single biggest killer and a leading cause of premature morbidity. The scientific toolkit for preventing and treating CVD has evolved dramatically in the last decade – yet everyday practice has not fully caught up. This article makes the case for a pragmatic but ambitious reset of UK CVD management with six mutually reinforcing moves:
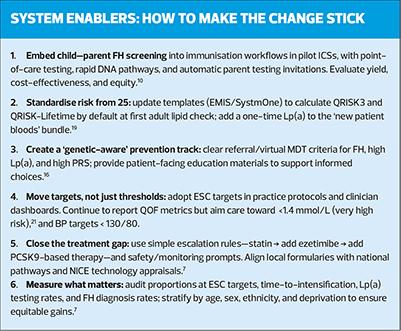
- Detect familial hypercholesterolaemia (FH) in childhood
- Start primary prevention risk assessment from age 25 using 10-year and lifetime risk
- Incorporate polygenic risk scores (PRS) where they add value
- Measure lipoprotein(a) [Lp(a)] at least once in adulthood
- In secondary prevention, aim for European Society of Cardiology (ESC) LDL-cholesterol targets rather than stopping at current NICE/QOF thresholds; and
- Aim for lower BP targets those with treated hypertension.
None of these steps is radical in isolation. Together, they are a coherent, evidence-anchored strategy to bend the UK CVD curve sooner, faster, and more fairly.
WHY CHANGE?
CVD risk starts early, CVD mortality is rising – and we’re catching it too late.
CVD mortality in the UK started to plateau from 2010 to 2019 and since 2019 has been rising for the first time in decades. CVD mortality is four times higher in the most deprived areas than in the least deprived.1,2 Furthermore, while patients with CVD in the most deprived areas are more likely to treated with lipid lowering therapy, they are less likely to have their cholesterol treated to target. The main contributory risk factors to death rates from cardiovascular diseases in the UK are high systolic blood pressure and high LDL cholesterol at estimated deaths rates of 43.9 per 100,000 and 21.6 per 100,000 respectively.3 CVD Prevent data from March 2025 shows that 14.47% of patients with atherosclerotic CVD (ASCVD) are not on lipid lowering therapy, and 51.75% are not at conservative NICE and QoF targets, let alone lower evidence-based ESC targets.4-6 We need to improve performance in these areas to help reverse the trend of rising CVD mortality and morbidity.
The pathogenesis of atherosclerosis begins decades before the first event. Traditional 10-year risk framing inevitably underestimates lifetime exposure in younger adults and delays meaningful intervention until risk is ‘mathematically visible.’ That delay is costly: cumulative LDL-cholesterol burden, genetically mediated risks (FH and polygenic risk), and high Lp(a) all act over time. Shifting detection and treatment earlier – without medicalising youth – means using tools that quantify lifetime risk, identifying those with genetically high risk sooner, and treating secondary prevention patients to levels that match modern outcome data. NICE NG238 updated risk guidance and lipid pathways in late 2023; implementation has progressed, but there are still major opportunities to go further where the evidence supports it.7
1.DETECT FAMILIAL HYPERCHOLESTEROLAEMIA IN CHILDHOOD
The case
Heterozygous FH affects roughly 1 in 250 people; in the UK an estimated 270,000 people live with FH and many remain undiagnosed. Early identification and treatment prevents premature coronary disease and normalises life expectancy. Yet UK primary care data show FH is still under-recognised, and many diagnosed patients remain undertreated.8
UK policy and practice today
NICE CG71 and Quality Standard QS41 recommend cascade testing and ensuring children at risk (because a parent has FH) are offered diagnostic tests by age 10. This is good – but it misses families where the parent is undiagnosed.9
Go earlier, go broader
Compelling evidence supports child–parent screening at routine immunisation visits (age 1–2). In a landmark NEJM study, finger-prick cholesterol in toddlers flagged likely FH; confirmatory DNA testing in flagged cases identified affected children and parents efficiently – screen one child, protect a family. Implementation pilots showed high acceptability in primary care. While the UK National Screening Committee (UK NSC) has not yet recommended universal child screening, primarily citing uncertainty over optimal age and thresholds, the balance of evidence and feasibility favours scaling pilots and integrating with immunisation contacts.10
Practical recommendation
Maintain cascade testing per NICE – but add child–parent screening pilots at 1–2 years in regions with lipid/DNA confirmatory pathways, with evaluation against agreed outcome metrics (detection yield, treatment initiation, family cascade completion). This will reveal undiagnosed parents and close the care gap earlier.11
2. START PRIMARY PREVENTION RISK ASSESSMENT FROM AGE 25
The case
Short-term calculators underestimate risk in younger adults. A 28-year-old with borderline blood pressure, rising BMI, family history and a suboptimal lipid profile may still have a low 10-year risk but a high lifetime risk – and a large preventable LDL ‘area-under-the-curve’ (cumulative risk over time). NICE NG238 explicitly recommends QRISK3 for people aged 25–84. Building on this, QRISK-Lifetime quantifies lifetime CVD probability and shows how modifiable factors change the trajectory. Used together, these tools support earlier lifestyle action and timely statin discussion in those with high lifetime risk – even when the 10-year risk is below treatment thresholds.5
What NICE and the charities say
NICE’s 2023 update confirms QRISK3 for 25–84-year-olds, and the British Heart Foundation (BHF) public guidance aligns with this age range, improving public understanding and clinical consistency. External validation work on QRISK-Lifetime is emerging, strengthening confidence in its use as a complementary lens.12
Practical recommendation
At first NHS Health Check (or opportunistically for those with risk factors) from age 25, record a basic lipid panel, blood pressure, smoking status, BMI/waist ratio, and family history; calculate both QRISK3 (10-year) and QRISK-Lifetime. Use lifetime risk to drive earlier lifestyle prescriptions, aggressive LDL-lowering in high-lifetime-risk phenotypes, and follow-up intervals proportionate to trajectory rather than age alone.5
3. ADD PRS TO SHARPEN PREVENTION IN SELECTED GROUPS
The case
Beyond rare monogenic FH, polygenic risk scores (PRS) aggregate multiple common genetic variants to quantify ASCVD risk that can rival FH-level event rates at the population tail. Large UK and multi-ancestry studies show modern PRS can improve discrimination and reclassification for CAD when layered onto conventional risk factors. People in the top few percent of a polygenic score often reach ‘average’ 50-year-old’s risk in their 30s, which argues for earlier preventive action when identified. PRS can help identify individuals without monogenic mutations but with equivalent or higher lifetime risk. The HEART pilot in the NHS showed that PRS integration into QRISK led to clinically significant risk reclassifications in 24 % of participants, with GPs intending to change management in ~13 % based on the new information.13 While further validation is needed, PRS has the potential to personalise primary prevention strategies and guide earlier use of advanced therapies in high-risk individuals.
Equity and calibration
Earlier PRS derived from European datasets had transferability issues; however, newer multi-ancestry scores demonstrate improved performance across ancestries by integrating genome-wide association study (GWAS) data from diverse populations and relevant risk factors. While ongoing calibration is essential, the trajectory is clear: PRS can responsibly augment risk assessment when used with – not instead of – clinical tools.14
Practical recommendation
Offer PRS in younger adults (e.g., 25–45) with borderline 10-year risk but strong family history or adverse lifetime risk profile; use results for ‘right-time’ statin initiation and intensity, framing decisions around absolute lifetime benefit. PRS should be integrated into structured pathways with genetic literacy, consent, and transparent communication of limitations.15
4. MEASURE Lp(a) ONCE IN ADULTHOOD AND INCLUDE IN TREATMENT PLANS
The case
Lp(a) is a genetically determined, largely non-modifiable atherothrombotic risk factor with lifetime exposure. Estimates suggest 20% of the UK population have raised Lp(a) levels rising to 25% on South Asians and 30% in black Africans. Large UK Biobank analyses show graded, independent associations between Lp(a) and ASCVD, while European and international consensus statements now recommend once-in-a-lifetime testing in most adults – earlier if family history or premature ASCVD is present. Elevated Lp(a) helps explain ‘residual risk’ and identifies people who may benefit from lower LDL-C targets and earlier therapy.16 (See Lipoprotein(a): the missing piece in the puzzle of residual cardiovascular risk, Practice Nurse 2025;55(3):27-30)
Guidance and evolving therapeutics
Current UK lipid pathways acknowledge Lp(a) as a risk modifier; the American College of Cardiology and European statements detail who to test and how to interpret results (i.e., thresholds such as ≥125–175 nmol/L as clearly high). Specific Lp(a)-lowering agents (antisense and siRNA) are in advanced trials; until then, ‘treat the treatable’: aim lower on LDL-C and non-HDL-C, intensify statins/ezetimibe/bempedoic acid/PCSK9-based therapy, and control other risk factors aggressively.17
Practical recommendation
Add a single Lp(a) measurement to the first adult lipid profile (and in any premature ASCVD, unexplained high risk, or suspected FH). Use the result to downshift LDL-C goals (e.g., target ESC levels), prioritise therapy intensity, and prompt family risk conversations.7
5. IN SECONDARY PREVENTION, TREAT TO ESC LDL-C TARGETS
The case
Outcome trials and contemporary meta-analyses show a near-linear relationship: every 1 mmol/L LDL-C reduction yields a roughly 20–25% relative risk reduction in major vascular events. The ESC/EAS 2019 guideline sets ambitious, evidence-proportionate goals for very-high-risk patients (most people with established ASCVD): LDL-C <1.4 mmol/L and ≥50% reduction from baseline (with <1.0 mmol/L considered in recurrent event patients and those with polyvascular disease). These goals are achievable today with high-intensity statins plus ezetimibe and/or PCSK9-based therapies, and they align with the biology of cumulative exposure and residual risk.6
Where the UK is now
NICE NG238 and NHS England pathways have moved in the right direction, supporting stepwise intensification and codifying non-HDL/LDL thresholds. However, QOF currently sets less stringent targets for incentives (i.e., LDL ≤2.0 mmol/L or non-HDL ≤2.6 mmol/L), which risks normalising undertreatment in exactly those who would benefit most from more intense LDL lowering. Aligning day-to-day practice with ESC targets would better reflect contemporary evidence.7
Practical recommendation
In all ASCVD patients:
- Start or up-titrate to high-intensity statin (atorvastatin 80 mg or rosuvastatin 20–40 mg) unless contraindicated.
- Add ezetimibe and bempedoic acid if needed if LDL-C remains ≥1.4 mmol/L (or non-HDL ≥2.2 mmol/L). If after full dose (or tolerated dose) high intensity statin LDL-C >/= 2.6 mmol/L my practice is to start Inclisiran first before adding ezetimibe or/and or Ezetimibe + bempedoic acid, to avoid locking patients out of Inclisiran eligibility.
- If still above target, add PCSK9-based therapy (mAb or Inclisiran per NICE TA) and re-measure at appropriate intervals until at target.
- Use ESC targets as the north star for clinical decisions and patient conversations; meet QOF thresholds on the way, but don’t stop there.7
6. IN HYPERTENSION. TREAT TO LOWER TARGETS
The case
High blood pressure (BP) is the leading modifiable risk factor for premature morbidity and mortality from CVD worldwide, and significantly contributes to chronic kidney disease (CKD), heart failure and dementia. Over the past 15 years, several large outcome trials have been conducted to specifically compare an intensive BP treatment target (systolic BP <120mmHg) with a less intensive target (SBP <140mmHg) in men and women, with and without pre-existing CVD and diabetes. These trials consistently demonstrate that aiming for an SBP treatment target <120 mmHg resulted in a greater reduction in major adverse cardiovascular events (MACE) compared with an SBP target <140mmHG, with no overall significant difference in reported serious adverse events between the treatment groups.18
Where the UK is now
Current NICE BP treatment targets for people aged under 80 years are <140/90, with <130/80 only recommended in specific clinical situations (i.e., some patients with diabetes and CKD). March 2025 CVD Prevent data show 29.69% of all patients and 32.65% of those under 80 years old achieve a target of <140/90.4
Practical recommendation
The British and Irish Hypertension Society recommends an on-treatment BP target <130/80 (measured by 7-day average home BP monitoring or day-time average ambulatory BP monitoring or office BP) or as low as reasonably achievable (ALARA) without causing unacceptable side-effects within 6 months of initiating treatment, among alladults with confirmed diagnosis of hypertension. Polypharmacy will often be needed to achieve these targets.18
PULLING IT TOGETHER: A PRACTICAL UK PATHWAY FOR 2025 AND BEYOND
At age 1–2: pilot child–parent screening for FH at immunisation visits, coupled with local confirmatory DNA and specialist referral capacity; continue cascade testing to age 10 for known-risk families.10
At age 25 and at first adult check-up: record lipids, blood pressure, smoking, BMI/waist, family history; calculate QRISK3 (10-year) and QRISK-Lifetime; measure Lp(a) once; consider PRS for those with strong family history or high lifetime trajectory.19
Treatment – primary prevention
- Anchor decisions in shared decision-making on lifetime benefit; when LDL-C is high or lifetime risk is high, start statin earlier and aim for meaningful absolute reductions.
- Use Lp(a) and PRS to justify earlier and more intensive LDL-C and/or non-HDL-C lowering in selected patients.14
- Manage hypertensive patients to BISH suggested target of less than 130/80.
Treatment – secondary prevention
- Escalate to ESC LDL-C targets (<1.4 mmol/L and ≥50% reduction, <1.0mmol/L if recurrent events while on statin-based therapy or polyvascular CVD), using combination therapy as needed; meet QOF thresholds as a staging post, not the destination.
- Embed this in practice protocols, audit loops, prescribing support tools and recall prompts.20
ADDRESSING COMMON CONCERNS
‘Won’t this over-medicalise young adults?’
No – starting at 25 does NOT mean universal statins. It means a better conversation informed by both short-term and lifetime risk. For many, this will motivate earlier lifestyle changes and periodic review. For a subset, i.e., those with very high LDL-C, FH, high PRS or Lp(a), it will appropriately bring forward treatment with the greatest lifetime benefit.5
‘Is PRS ready for routine care?’
PRS is not a general screening test for everyone yet. But in selected younger adults –especially those with family history or ambiguous risk – it can clarify trajectory. Newer multi-ancestry scores improve performance across ethnicities, and UK systems can adopt PRS through carefully governed pilots that measure clinical impact and equity.
‘Is Lp(a) actionable without specific drugs?’
Yes. Elevated Lp(a) re-stratifies risk and justifies lower LDL-C and non-HDL-C goals and tighter control of other factors. Dedicated Lp(a)-lowering agents are on the horizon; knowing who stands to benefit prepares services and patients for timely access.17
‘Are ESC targets too hard to reach?’
Most patients can reach <1.4 mmol/L with combination therapy. NHS England’s lipid optimisation pathway already embeds stepwise intensification that aligns with this approach; the gap is ambition, not tools.7
THE PAYOFF: EARLIER PREVENTION, FEWER EVENTS, BETTER EQUITY
When risk is quantified earlier and genetic contributors are acknowledged, preventive therapy can be smarter and fairer. Data from genomic screening programs suggest that identifying high-risk individuals sooner shifts preventive care forward by years, a window in which cumulative exposure is modifiable and events are avoidable. Combining lifetime risk, Lp(a), PRS, and FH detection changes who gets help when – and aligns secondary prevention with the levels supported by modern outcomes trials.
CONCLUSION
CVD prevention is a timing problem. The UK has the tools to solve it:
- Find FH in childhood, not after the first MI.
- Assess risk from 25, using both 10-year and lifetime perspectives.
- Layer in genetics –PRS for selected younger adults and one-time Lp(a) for all – to uncover hidden risk.
- In established disease, treat to ESC targets, not just to the QOF line in the sand.
This is not overreach; it is a rational update to match the evidence. If we act now, the next decade can bring fewer heart attacks and strokes, narrower inequalities, and lives prolonged not just by years, but by healthy years.
References
- Marmot M, Allen J, Boyce T, et al. The Marmot Review 10 years on; 2020. Institute of Health Equity. https://www.instituteofhealthequity.org/resources-reports/marmot-review-10-years-on
- Department of Health and Social care. Lord Darzi’s Independent investigation of the NHS in England; September 2024. https://www.gov.uk/government/publications/independent-investigation-of-the-nhs-in-england
- Institute for Health Metrics and Evaluation (IHME). Global Burden of Disease 2024. https://www.healthdata.org/research-analysis/gbd
- Cardiovascular disease Prevention Audit (CVDPREVEVENT); March 2025 data. https://data.cvdprevent.nhs.uk/data-explorer
- NICE NG238. Cardiovascular disease: risk assessment and reduction, including lipid modification; 2023. https://www.nice.org.uk/guidance/ng238
- Mach F, Koskinos K, Roeters JE, et al. 2025 Focused Update of the 2019 ESC/EAS Guidelines for the management of dyslipidaemias. European Heart Journal, ehaf190; 29 August 2025. https://doi.org/10.1093/eurheartj/ehaf190
- NHS England. Lipid optimisation pathway: secondary prevention in primary care and the community; 2024. https://www.england.nhs.uk/long-read/lipid-optimisation-pathway-secondary-prevention-in-primary-care-and-the-community/
- British Heart Foundation. Familial Hypercholesterolaemia; March 2023. https://www.bhf.org.uk/informationsupport/conditions/familial-hypercholesterolaemia
- NICE CG71. Familial Hypercholesterolaemia: identification and management; 2008, updated October 2024. https://www.nice.org.uk/guidance/cg71
- Wald DS, et al. Child-Parent Familial Hypercholesterolaemia screening in primary care. NEJM 2016; 375: 1628-1637. Doi:10.1056/NEJMoa 1602777
- Public Health England. Familial Hypercholesterolaemia: Implementing a systems approach to its detection and management; 2018. https://www.england.nhs.uk/london/wp-content/uploads/sites/8/2019/11/Familial-Hypercholesterolaemia-Implementation-Guide.pdf
- British Heart Foundation. QRisk: how it works and what your score means. July 2025; https://www.bhf.org.uk/informationsupport/heart-matters-magazine/medical/qrisk
- Fuat A, Adlan E, Monane M, et al A polygenic risk score added to a QRISK2 cardiovascular disease risk calculator demonstrated robust clinical acceptance and clinical utility in the primary care setting. Eur J Prevent Cardiol 2024;31(6):716-722 https://doi.org/10.1093/eurjpc/zwae004 (https://doi.org/10.1093/eurjpc/zwae004)
- Patel AP, et al. A multi-ancestry polygenic risk score improves risk prediction of coronary artery disease. Nat Med 2023;29:1793-1803
- Manikpurage HD, et al. Polygenic risk score for coronary artery disease improves the prediction of early onset myocardial infarction in men. CicGenomPrecisMed 2021 Dec: 14(6). Doi:10.116/CIRCGEN.121.003452
- Kronenberg F, Mora S, Stroes ESG, et al. Lipoprotein(a) in atherosclerotic cardiovascular diseaseand aortic stenosis: a European Atherosclerosis Society (EAS) consensus statement. Eur Heart J 2022;43(39):3925-46 https://doi.org/10.1093/eurheary/ehac361 (https://doi.org/10.1093/eurheary/ehac361)
- Alebna PL, Mehta A. An update on Lipoprotein(a): the latest on testing, treatment and guideline recommendations; September 2023. American College of Cardiology. https://www.acc.org/Latest-in-Cardiology/Articles/2023/09/19/10/54/An-Update-on-Lipoprotein-a
- Faconti L, Tantirige NRm George J, et al. Call to action: British and Irish Hypertension Society position statement on blood pressure treatment thresholds and targets. J Human Hyperten 2025;39:537-540. https://doi.org/10.1038/s41371-025-01055-z (https://doi.org/10.1038/s41371-025-01055-z)).
- ClinRisk, QRISK3-lifetime. https://qrisk.org/lifetime/ (https://qrisk.org/lifetime/)
- NHS England. Quality and Outcomes Framework guidance 2025/26; March 2025. https://www.england.nhs.uk/publication/quality-and-outcomes-framework-guidance-for-2025-26/
- Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guideline for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41(1):111-188. Doi:10.1093/eurheart/ehz455
Related articles
View all Articles