Lipoprotein(a): the missing piece in the puzzle of residual cardiovascular disease risk?
Mohammed Elmelesy, MBChB MSc MRCP | Fiona Connolly, MA MB BChi | Professor Derek Connolly, BSc[Hons] MBChB PhD DSc FRCP, FRCPE FESC
Mohammed Elmelesy, MBChB MSc MRCP
The Midland Metropolitan University Hospital (Sandwell and West Birmingham Hospitals NHS Trust)
Fiona Connolly,MA MB BChir
Addenbrookes Hospital, Cambridge
Professor Derek Connolly,BSc[Hons] MBChB PhD DSc FRCP, FRCPE FESC
The Midland Metropolitan University Hospital (Sandwell and West Birmingham Hospitals NHS Trust); Aston University Medical School; University of Birmingham, Institute of Cardiovascular Sciences
Despite lowering LDL-C to optimum levels there is still a residual risk of cardiovascular events, which is thought to be due to another lipoprotein, Lp(a). The hunt is underway for ways to lower Lp(a) and thus reduce cardiovascular risk
TAKE HOME POINTS
- Sometimes described as a silent risk factor for cardiovascular disease, raised Lp(a) levels may explain residual risk in people whose LDL-C levels have been optimised
- High Lp(a) levels are particularly dangerous in smokers, and in people with diabetes, hypertension and/or obesity
- Genetic studies suggest that lowering Lp(a) will reduce cardiovascular risk
- Most existing lipid lowering therapies have minimal effects on Lp(a) levels
- Trials are underway of a number of specific Lp(a) inhibitors that may help to reduce residual CVD risk
Worldwide, over 18 million deaths occur each year due to cardiovascular disease and it is well established that cholesterol metabolism and in particular apolipoprotein B-containing blood lipoproteins increase the risk of atherosclerosis and CVD.1,2 LDL-Cholesterol is the most abundant of these apolipoprotein B containing lipoproteins and a plethora of studies using statins, bempedoic acid, ezetimibe and PCSK9 targeted therapy have shown that reducing LDL-C results in a significant reduction in cardiovascular disease events and mortality.3
Despite lowering LDL-C to optimum levels there is still a residual risk of cardiovascular events and a significant component of this is thought to be due to lipoprotein (a), usually shortened to ‘Lp(a)’, and this has provoked a search for specific inhibitors of Lp(a).4
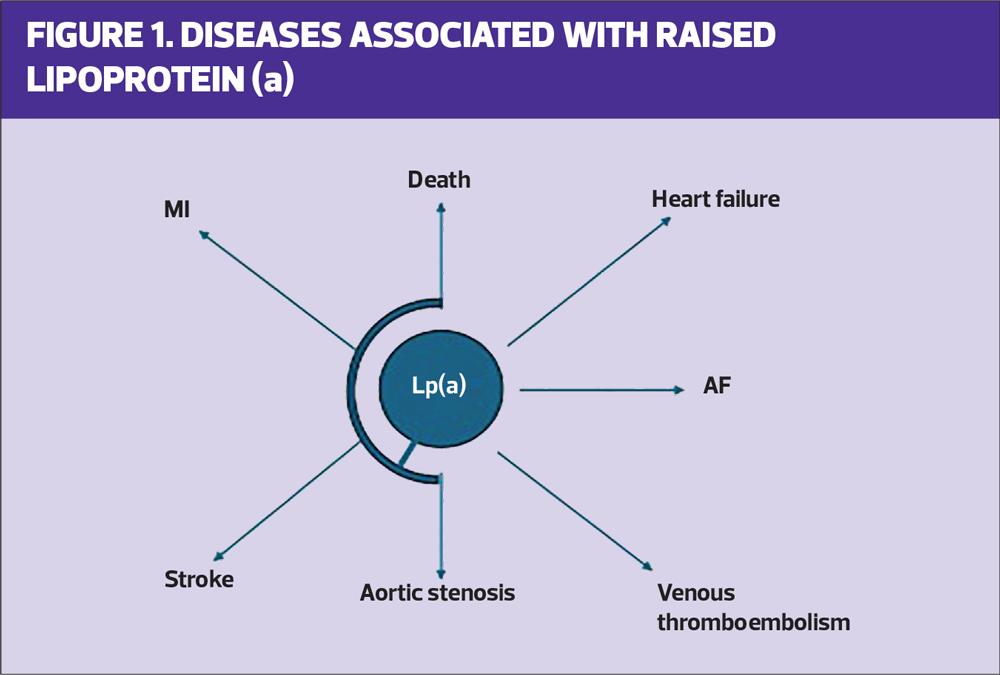
Lp(a) was discovered in 1963 and, like LDL-C, is also an apolipoprotein B containing ‘LDL-C-like’ particles. It is present in all humans and higher primates only although the hedgehog has a similar molecule. Lp(a) is related to the clotting gene plasminogen, and is involved in both prothrombotic and proinflammatory pathways.5,6 A high Lp(a) level is associated with higher levels of cardiovascular disease, particularly myocardial infarction and stroke. It is also associated with calcific aortic stenosis, heart failure, atrial fibrillation and premature death. There is a linear increase in CVD risk as the Lp(a) rises.7
There are two ways to measure Lp(a) in either mg/dL or in nmol/L and the latter is generally preferred in the UK but most of the research studies use mg/dL which remains preferred in the US. Although there is no generally accepted consensus on overall risk thresholds, the risk of cardiovascular disease rises from levels of around 30 mg/dL which is equivalent to 75 nmol/L.
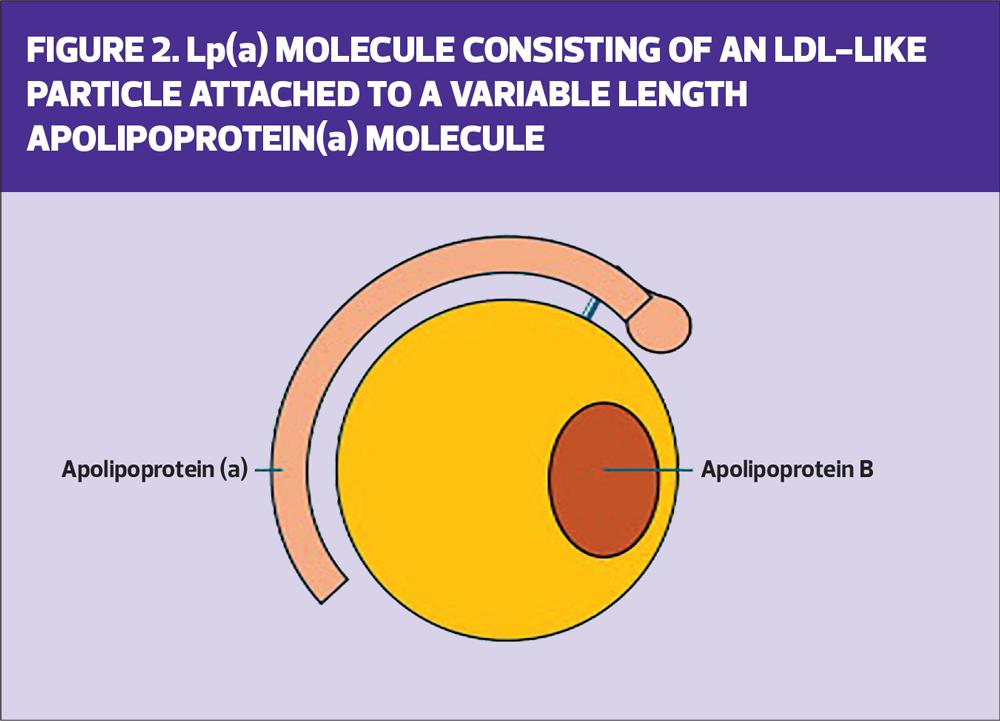
Lp(a) is synthesised in the liver and comprises a large ball of lipid, containing apolipoprotein B.
Lp(a) levels are genetically inherited (with a gene from each parent) and up to one-third of the world’s population may have raised Lp(a) levels, with higher levels in some parts of the world. In particular, patients with a West African or South Asian heritage may have higher level levels of Lp(a), which gives them a strong family history of cardiovascular disease.9 If you find a patient with a raised Lp(a) it is likely their genetically close relatives may also have a raised Lp(a), and therefore family screening is likely to become normal as it is with familial hyperlipidaemia due to raised LDL-C.
Unlike LDL-C, which can be increased by lifestyle factors, Lp(a) levels are relatively consistent throughout life from the age of 15 months to the age of 85.10 There is a small increase in women at the time of the menopause, but levels can also rise during periods of inflammation, or in liver disease or kidney disease, and may contribute to the increase in cardiovascular events seen in people with these conditions. Higher Lp(a) levels are particularly dangerous in smokers, people with diabetes, hypertension and obesity, as raised Lp(a) multiplies the risk in combination with these other risk factors.
In the large biobank studies such as the UK Biobank, genetic studies suggest that if you are lucky and inherit genes that results in a lower level of Lp(a), this confers a much lower level of cardiovascular disease with no other additional deficits or ‘price to pay’. This therefore strongly suggests that lowering Lp(a) with specific inhibitors will lower the risk of CVD and premature death beyond that achievable with LDL-C reduction.12,13
Existing therapies for LDL-C reduction have varying effects on Lp(a). Statins may increase Lp(a) levels slightly, while bempedoic acid and ezetimibe have neutral effects on Lp(a). On the other hand, PCSK9 targeted therapies such as inclsiran and evolocumab reduce Lp(a) by 15-40%.7 The trials of PCSK9 therapies, such as FOURIER,14 showed a large reduction in total cardiovascular events on top of statin therapy with very low LDL cholesterols. While the low LDL-C is likely to have caused the reduced number of events, it is possible that the 15% reduction in Lp(a) may also have contributed.
Similarly, obicetrapib, a CETP inhibitor in development, lowers Lp(a) by 50% but also LDL by 40%.15 It is currently being studied in the PREVAIL CVD outcomes trial (CVOT), expected to report in 2026. Lipoprotein apheresis, a treatment that removes cholesterol from the blood using a type of dialysis, reduces Lp(a) by up to 75% and may reduce cardiovascular events in observational data, but more randomised trials are needed for definitive conclusions.16 Clearly, all these therapies also reduce other potentially harmful lipid subfractions so it’s difficult to tease out whether specific inhibition of Lp(a) has a definitive, separate benefit. Analysis of the data from the Biobank genetic studies suggest that with each 5 mg/dL reduction in Lp(a) there will be a 2.5% reduction in cardiovascular events.17
Lp(a) INHIBITION
At present, there are five specific inhibitors of Lp(a) in development. Pelacarsen, (Novartis), is a subcutaneous injectable antisense oligonucleotide therapy, which, with monthly injections, lowers Lp(a) levels by 70-88%.18 This is currently being studied in the large phase 3B Lp(a) HORIZON randomised, double blind, placebo controlled, multi-centre cardiovascular outcomes trial, which is expected to present in Spring 2026. This will be the first trial to tell us whether inhibition of Lp(a) gives additional and independent benefits to LDL-C reduction. As well as having raised Lp[a], the study participants have to have had a prior history of either myocardial infarction, stroke or significant peripheral arterial disease, so this is a secondary prevention trial.19
There are three development programmes ongoing with small interfering RNAs against Lp[a].20 These small interfering RNAs, injected subcutaneously, could produce more convenient Lp(a) reduction than the monthly injections with pelacarsen,21 in the same way that inclisiran, a six-monthly small interfering RNA injection to block PCSK9, is more convenient than the monthly dosing schedule for the antibody evolocumab.
Olpasiran, (Amgen) is being studied in the large Phase 3B OCEAN(a) (Olpasiran Trials of Cardiovascular Events and Lipoprotein(a) Reduction) trial and is expected to present around 2028. Lepodisaran (Eli Lilly), and Zerlasiran (Silence Therapeutics) are at an earlier stage of development.22,23
Muvalaplin, (Eli Lilly), is an orally administered medication taken daily which can lower Lp(a) by up to 80%. In a recent phase 2 trial, it was well tolerated in patients at high risk of cardiovascular events.24 Muvalaplin will be studied in a planned phase 3 cardiovascular outcome trial in the near future.
AORTIC VALVE STENOSIS
Beyond ASCVD, the strongest causal association between high Lp(a) and any cardiovascular (CV) endpoint has been observed with calcific aortic valve stenosis, where the aortic valve is narrowed by the build-up of calcium. This is most prevalent form of valvular heart disease and aortic stenosis valve progression, and trials are being planned with the five specific inhibitors. Given the pandemic of rising levels of calcific aortic stenosis, and the high cost of replacing these valves, any therapy that reduces the need for valve replacement may potentially be extremely useful in reducing both morbidity and cost.25
The European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) recommends that every adult undergo Lp(a) testing at least once in their lifetime.26 If the results of clinical trials of specific inhibitors are as expected, then this will provide treatment options for patients previously diagnosed with coronary artery disease and raised Lp[a]. However, until licensed treatments become available, the good news is that if patients optimise all of their other risk factors, even with a high level of Lp(a) they may not develop any cardiovascular disease.27
WHAT CAN GENERAL PRACTICE NURSES DO?
- Test Lp(a) levels in all adults at each once: Lp(a) levels do not vary appreciably over a lifetime and there is a continuous association between raised Lp(a) and cardiovascular outcomes10
- Test Lp(a) levels in first degree relatives of people:
- With familial hypercholesterolaemia
- Family or personal history of very high Lp(a) or premature atherosclerotic cardiovascular disease
- Implement early and intensive management of other risk factors for CVD
- In the absence of licensed treatment options, people with very high Lp(a) and progressive CVD may benefit from lipoprotein apheresis
SUMMARY
In summary, Lp(a) is an atherogenic lipoprotein that increases the risk of CVD and aortic stenosis independent of LDL-C. Genetic studies suggest that lowering levels will reduce cardiovascular risk. Several specific inhibitors are in late-stage development with the results of the cardiovascular outcome trials expected in the next couple of years. If these trials are positive, this will result in a new pillar of therapy to protect patients against cardiovascular events. In particular, if the Lp(a) HORIZON Study is positive in 2026,19 this will open a whole new field in prevention of cardiovascular disease.
REFERENCES
- Shapiro MD, Fazio S. Apolipoprotein B-containing lipoproteins and atherosclerotic cardiovascular disease. F1000Res 2017;6:134.
- Wazir M, Olanrewaju OA, Yahya M, et al. Lipid Disorders and Cardiovascular Risk: A Comprehensive Analysis of Current Perspectives. Cureus 2023.31;15(12):e51395.
- Mhaimeed O, Burney ZA, Schott SL, et al. The importance of LDL-C lowering in atherosclerotic cardiovascular disease prevention: Lower for longer is better. Am J Prev Cardiol 2024;18:100649.
- Thanassoulis G. Screening for high lipoprotein(a). Circulation 2019;139(12):1493-1496.
- Konieczyńska M, Natorska J, Ząbczyk M, et al. Lipoprotein(a) and thromboembolism: current state of knowledge and unsolved issues. Arch Med Sci 2024;20(6):1770-1783.
- Dzobo KE, Kraaijenhof JM, Stroes ESG, et al. Lipoprotein(a): An underestimated inflammatory mastermind. Atherosclerosis 2022;349:101-109.
- Vinci P, Di Girolamo FG, Panizon E, et al. Lipoprotein(a) as a Risk Factor for Cardiovascular Diseases: Pathophysiology and Treatment Perspectives. Int J Environ Res Public Health. 2023;20(18):6721.
- Bess C, Mehta A, Joshi PH. All we need to know about lipoprotein(a). Prog Cardiovasc Dis 2024;84:27-33.
- Reyes-Soffer G. The impact of race and ethnicity on lipoprotein(a) levels and cardiovascular risk. Curr Opin Lipidol 2021;32(3):163-166.
- Raitakari O, Kivelä A, Pahkala K, et al. Long-term tracking and population characteristics of lipoprotein (a) in the Cardiovascular Risk in Young Finns Study. Atherosclerosis 2022;356:1827.
- Enkhmaa B, Berglund L. Non-genetic influences on lipoprotein(a) concentrations. Atherosclerosis 2022;349:53-62.
- Deshotels MR, Sun C, Nambi V, et al. Temporal trends in lipoprotein(a) concentrations: the atherosclerosis risk in communities study. J Am Heart Assoc 2022;11:e026762.
- Rikhi R, Hammoud A, Ashburn N, et al. Relationship of low-density lipoprotein cholesterol and lipoprotein(a) to cardiovascular risk: the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis 2022;363:102–8.
- O'Donoghue ML, Fazio S, Giugliano RP, et al. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk (FOURIER trial). Circulation 2019;139(12):1483-1492.
- Tsimikas S, Yang X, Hsieh A, et al. Obicetrapib demonstrates significant reductions of Lp(a) on top of high-intensity statins Atherosclerosis 2024;395(1)118412.
- Waldmann E, Parhofer KG. Lipoprotein apheresis to treat elevated lipoprotein (a). J Lipid Res 2016;57(10):1751-1757.
- Doherty S, Hernandez S, Rikhi R, Mirzai S, et al. Lipoprotein(a) as a Causal Risk Factor for Cardiovascular Disease. Curr Cardiovasc Risk Rep 2025;19(1):8.
- Graham MJ, Viney N, Crooke RM, et al. Antisense inhibition of apolipoprotein(a) to lower plasma lipoprotein(a) levels in humans. J Lipid Res. 2016;57(3):340-351.
- ClinicalTrials.gov. A randomized double-blind, placebo-controlled, multicenter trial assessing the impact of lipoprotein (a) lowering with pelacarsen (TQJ230) on major cardiovascular events in patients with established cardiovascular disease (HORIZON)NCT04023552; 2019. https://classic.clinicaltrials.gov/ct2/show/NCT04023552
- Thau H, Neuber S, Emmert MY, et al. Targeting Lipoprotein(a): Can RNA therapeutics provide the next step in the prevention of cardiovascular disease? Cardiol Ther 2024;13(1):39-67.
- O'Donoghue ML, Rosenson RS, López JAG, et al. The off-treatment effects of olpasiran on lipoprotein(a) lowering: OCEAN(a)-DOSE extension period results. J Am Coll Cardiol 2024;84(9):790-797.
- Nissen SE, Linnebjerg H, Shen X, et al. Lepodisiran, an extended-duration short interfering RNA targeting lipoprotein(a): a randomized dose-ascending clinical trial. JAMA 2023; 330(21):2075-2083.
- Nissen SE, Wolski K, Watts GF, et al. Single ascending and multiple-dose trial of zerlasiran, a short interfering RNA targeting lipoprotein(a): a randomized clinical trial. JAMA 2024;331(18):1534-1543.
- Nicholls SJ, Ni W, Rhodes GM, et al. Oral Muvalaplin for Lowering of Lipoprotein(a): A Randomized Clinical Trial. JAMA 2025;333(3):222-231.
- Tsimikas S. Lp(a) and accelerated progression of aortic stenosis: a rationale for universal measurement and therapeutic targeting. JACC Asia 2024;4(10):761-763.
- Kronenberg F, Mora S, Stroes ESG, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J 2022;43:3925–46.
- Razavi AC, Reyes MP, Wilkins JT, et al. Traditional risk factors, optimal cardiovascular health, and elevated lipoprotein(a). Eur J Prev Cardiol 2024 Nov 28:zwae382.