COPD: a guideline update
Practice Nurse
INTRODUCTION
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023 report represents a major revision of the 2022 report, with a new definition of chronic obstructive pulmonary disease (COPD), a new assessment tool, and new information on the choice of inhaler device.
COPD is one of the top three causes of death worldwide, and many people suffer from the disease for years and die prematurely from it or its complications. The burden of COPD is projected to increase over the coming years because of continued exposure to COPD risk factors and population ageing.
LEARNING OBJECTIVES
On completion of this module, you should have a better understanding of:
- Key changes in the GOLD 2023 report
- The symptoms of, and diagnostic criteria for COPD
- The ABE assessment tool
- GOLD’s recommendations for initiation of treatment and follow-up
This resource is provided at an intermediate level. Read the accompanying article, which provides further detail and management options, before answering the self-assessment questions, and reflecting on what you have learned.
Complete the resource to obtain a certificate to include in your revalidation portfolio. You should record the time spent on this resource in your CPD log.
The latest COPD guideline from the Global Initiative for Chronic Obstructive Lung Disease (GOLD) represents a major revision of the 2022 report. It includes a new definition of chronic obstructive lung disease, a simplified assessment tool with greater emphasis on the importance of exacerbations, and more detailed information of the choice of inhaled medication and devices.
GOLD has clarified the definition of COPD as a heterogenous lung condition, characterised by chronic respiratory symptoms:
- Dyspnoea
- Cough
- Sputum production, and
- Exacerbations
Symptoms are due to abnormalities of the airways (bronchitis, bronchiolitis) and/or alveoli (emphysema) that cause persistent, often progressive, airflow obstruction.
DIAGNOSTIC CRITERIA
The presence of non-fully reversible airflow limitation (FEV1/FVC
Presentation
Patients with COPD typically complain of breathlessness, activity limitation, and/or cough with or without sputum production, and they may experience exacerbations (acute events with increased respiratory symptoms) that affect their health status and prognosis, and which need specific prevention and treatment. Clinical indicators of COPD are shown in Table 2 (additional tables can be viewed in COPD: GOLD 2023).
Opportunities
Extensive under- and misdiagnosis of COPD leads to patients receiving incorrect treatment or no treatment at all. Recognising that environmental factors other than smoking can lead to COPD, that it can start early in life and affect young people, and that there are precursor conditions presents new opportunities for prevention, early diagnosis, and prompt and appropriate treatment.
DIAGNOSIS
A diagnosis of COPD should be considered in any patient who has dyspnoea, chronic cough or sputum production, a history of lower respiratory tract infections and/or a history of exposure to risk factors for the disease, but GOLD says that the presence of a post-bronchodilator FEV1/FVC <0.7, confirmed on spirometry, is mandatory to establish the diagnosis of COPD.
Chronic dyspnoea is the cardinal symptom of COPD, while cough with sputum production occurs in around 30% of patients. Symptoms may vary over time, and may be present for many years before the development of airflow obstruction. Dyspnea should be measured by the 5-level modified Medical Research Council (mMRC) scale.
Airflow obstruction may also occur without breathlessness or cough and sputum production, and vice versa.
Chronic cough is often the first symptom of COPD, but may be dismissed by the patients as an expected consequence of smoking or other environmental exposures.
Patients with COPD commonly produce sputum with coughing, but this may be difficult to quantify because patients may swallow it rather than expectorating it. Sputum production can be intermittent, with periods of flare-up and remission. The presence of purulent sputum suggests an increase in inflammatory mediators and may identify the onset of a bacterial exacerbation.
GOLD uses FEV1/FVC <0.7 as the diagnostic criterian, but warns that using a fixed ratio may result in over-diagnosis in the elderly, and underdiagnosis in young adults, especially in mild disease, compared with using a cut-off based on lower limit of normal (LLN) values. However, there are no studies validating the use of LLN in populations where smoking is not the major cause of COPD.
Assessment of airflow obstruction based on a single measurement of the post-bronchodilator FEV1/FVC ratio should be confirmed by repeat spirometry if the value is between 0.6 and 0.8. Assessing the degree of reversibility of airflow obstruction (i.e. measuring FEV1 before and after bronchodilator or corticosteroids) to inform treatment decisions is no longer recommended.
ASSESSMENT
Once the diagnosis of COPD has been confirmed by spirometry, assessment to guide therapy should focus on:
- Severity of airflow limitation
- Nature and severity of current symptoms
- Previous history of moderate and severe exacerbations
- Presence and type of other diseases.
The initial COPD assessment aims to establish the severity of airflow obstruction, how the disease affects the patient’s health status and to predict the risk of future exacerbations, hospital admissions, or even death, to select appropriate treatment.
Because there is only a weak correlation between severity of airflow obstruction and symptoms, formal assessment of symptoms using validated questionnaires is also needed.
GOLD recommends the use of the COPD Assessment Test (CATTM) – an 8 item questionnaire that is used to assess health status in patients with COPD. The score ranges from 0 to 40.
Additional clinical assessment, including the measurement of lung volumes, diffusion capacity, exercise testing and/or lung imaging may be considered in COPD patients with persistent symptoms after initial treatment. GOLD recommends chest CT imaging should be considered for patients with:
- Persistent exacerbations
- Symptoms out of proportion to disease severity on lung function testing
- FEV1 less than 40% predicted with significant hyperinflation, or
- For those who meet criteria for lung cancer screening.
The ABE assessment tool
GOLD has revised its ABCD assessment tool, and now recommends the use of a new, slightly simpler version, the ABE assessment tool (Table 5).
The latest evolution of the ABCD combined assessment tool reflects the clinical importance of exacerbations independent of the patient’s symptoms. The A and B groups remain unchanged but the C and D groups are merged into a single group E.
PHARMACOTHERAPY
Pharmacological treatment can reduce COPD symptoms, reduce the frequency and severity of exacerbations, and improve health status and exercise tolerance.
Each regimen should be tailored to the individual and guided by the severity of symptoms, risk of exacerbations, side effects, and comorbidities, as well as the patient’s response, preference and ability to use their inhaler.
Patients with COPD should be offered vaccinations for flu and pneumococcus (to decrease the incidence of lower respiratory tract infections); COVID-19; and, if not vaccinated in adolescence, the triple pertussis, tetanus and diphtheria vaccination.
Bronchodilators
Inhaled bronchodilators are central to symptom management and are usually given on a regular basis to prevent or reduce symptoms. Regular and as needed use of short-acting beta agonists (SABA) and short-acting antimuscarinics (SAMA) improves FEV1 and symptoms. Combinations of SABA and SAMA are superior to either medication alone in improving lung function and symptoms.
Long-acting beta agonists (LABA) and long-acting antimuscarinics (LAMA) significantly improve lung function, breathlessness, and health status, and reduce exacerbation rates, but LAMAs have a greater effect on exacerbation reduction compared with LABAs, and decrease hospitalisations. Using them in combination increases FEV1 and reduces symptoms compared with monotherapy, and is also more effective at reducing exacerbations than either alone. A combination inhaler may be more convenient for patients, and more effective, than multiple inhalers.
Inhaled corticosteroids (ICS)
Most studies have found that regular treatment with ICS alone does not alter the long-term decline in FEV1 or mortality in patients with COPD. Regular ICS treatment increases the risk of pneumonia, especially in patients with severe disease. An ICS combined with a LABA is more effective than the individual components in improving lung function and health status, and reducing exacerbations, in patients with moderate to very severe COPD. And triple therapy with LABA+LAMA+ICS is more effective than LABA+ICS, LABA+LAMA or LAMA monotherapy.
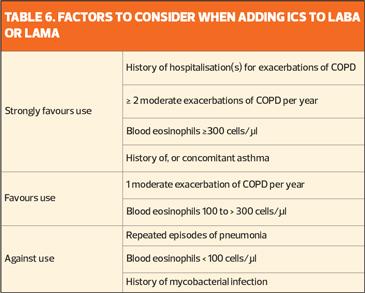
A number of studies have shown that blood eosinophil counts predict the efficacy of ICS (added to regular maintenance bronchodilator treatment) in preventing future exacerbations. No effect, or only a small effect, is seen when blood eosinophil count is <100 cells/μl, so this threshold can be used to identify patients who are unlikely to benefit from ICS, while those with a blood eosinophil count is ≥300 cells/μl are most likely to benefit (Table 6).
INHALED THERAPY
When treatment is given via inhaler, the importance of education and training in inhaler device technique cannot be overstated.
Worldwide, there are at least 33 different inhaled therapies – bronchodilators, short or long acting and ICS, alone or in combination – and at least 22 different inhaler devices are available, including metered dose inhalers (with or without a spacer), breath-actuated inhalers, soft mist inhalers and dry powder inhalers, and nebulisers. No device is available that avoids the need to explain, demonstrate and regularly check inhaler technique.
Selecting the optimum delivery system is essential to ensure that patients gain maximum benefit from their treatment: shared decision making has been shown to improve outcomes for patients with asthma, and is likely to do so for those with COPD.
Adherence
Adherence to inhaled medication is generally low, even in very severe disease. Factors such as the presence of comorbidities, especially depression, smoking status, education, disease severity and drug regimen (complexity and side effects) are the main causes of low adherence. Better patient understanding of their disease and medication can help to improve adherence, and behavioural approaches targeted to the individual’s barriers (e.g. keeping medication in one place, self-monitoring symptoms, medication reminders) are more effective than offering general suggestions.
INITIATING TREATMENT
GOLD has proposed a new algorithm for the initiation of pharmacological treatment according to the individualised assessment of symptoms and exacerbation risk, following the ABE assessment scheme (Table 7).
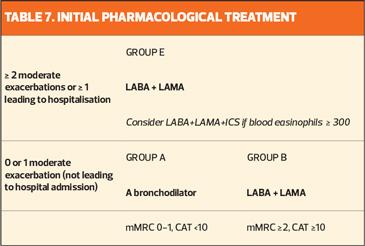
All Group A patients should be offered a bronchodilator, based on its effect on breathlessness. This can be either a short- or long-acting bronchodilator, although a long-acting bronchodilator is preferred except in patients with only very occasional breathlessness.
Patients in Group B should be started on a LABA+LAMA combination. These patients are likely to have comorbidities that increase their symptom burden and can affect their prognosis, so these should be investigated and treated according to current guidelines.
Group E patients should also be offered a LABA+LAMA combination. If there is an indication for ICS, LABA+LAMA+ICS is the preferred treatment option. However, the effect of ICS on exacerbation prevention correlates with eosinophil count, so there is an argument for reserving this treatment option for patients with a high eosinophil count (≥ 300 cells/ μl).
FOLLOW-UP
After starting any treatment, patients should be reassessed for attainment of treatment goals and identification of any barriers to successful treatment, following which, adjustments to treatment may be needed. Follow up should comprise:
- Review: review symptoms (dyspnoea) and exacerbation risk (previous history, eosinophils)
- Assess: assess inhaler technique and adherence, and non-pharmacological strategies (smoking cessation, physical activity and pulmonary rehabilitation, vaccination)
- Adjust: adjust drug treatment, including escalation or de-escalation as indicated; switching inhaler device or using a different long-acting bronchodilator may be appropriate. Any change of treatment requires a subsequent review of clinical response.
Additional tables and detail are available in our Guidelines in a Nutshell summary of the GOLD 2023 report, including GOLD grades of severity of airflow obstruction and CAT Assessment.
Reference
Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023 Report. 2 December 2022. https://goldcopd.org/2023-gold-report-2/