The UK Immunisation Schedule
Public Health England
Public Health England
April 2019
The Green Book chapter on the UK Immunisation Schedule has been comprehensively revised and updated and now includes the latest schedule and essential information about intervals between live vaccines.
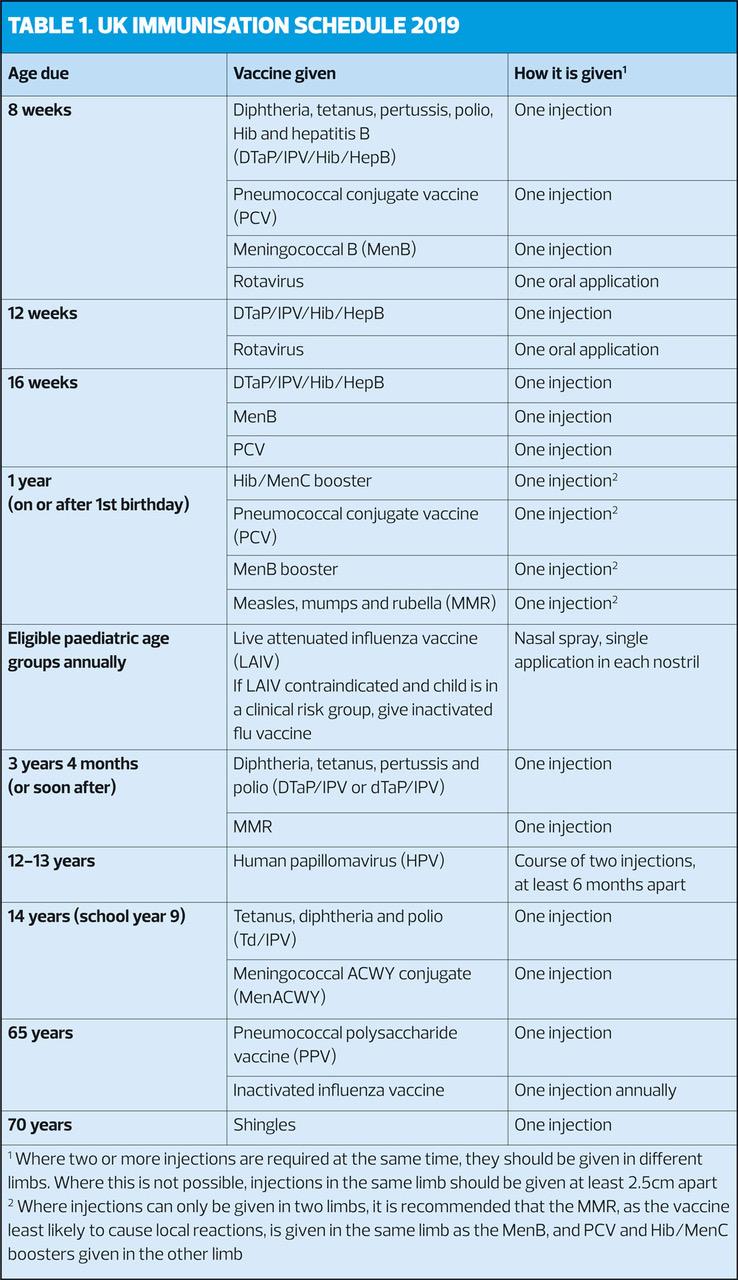
The UK immunisation schedule aims to provide early protection against diseases that are most dangerous for the very young, particularly diseases such as whooping cough, rotavirus and those due to pneumococcal, Hib and meningococcal infections. Further vaccinations are offered throughout life when people reach an age where they can derive the most benefit or where the programme will provide optimal control of the disease for the whole population.
Recommendations for the age at which vaccines should be given are made according to the age-specific risk for the disease, the risk of complications, the ability to respond to the vaccine, and the impact on spread in the population – so it is important to follow the schedule (Table 1) as closely as possible.
The schedule covers the following vaccine-preventable infections:
- Diphtheria
- Haemophilus influenzae type b (Hib)
- Hepatitis B
- Human papillomavirus (some strains)
- Influenza
- Measles
- Meningococcal disease (some serogroups)
- Mumps
- Pertussis
- Pneumococcal disease (some strains)
- Polio
- Rotavirus
- Rubella
- Shingles
- Tetanus
HPV for boys
From September 2019, boys aged 12-13 years (school year 8) will be eligible for the HPV vaccine for the first time. This aims not only to prevent HPV-related cancers in boys - notably penile, genital and anal cancers and some head and neck cancers, but also to increase herd immunity among girls. It is estimated that the HPV vaccine currently used will have prevented almost 100,000 cancers by 2058, when the people who were vaccinated as teenagers reach the age groups that would typically be affected by HPV-related cancers.
SCHEDULE FLEXIBILITY
The schedule is based on minimum intervals between doses of vaccines, recommended by the Joint Committee on Vaccination and Immunisation (JCVI).In health individuals, increasing the interval usually leads to a more pronounced response to the later dose.
- If a course of immunisation is interrupted there is no need to start again – the course should simply be resumed and completed as soon as possible.
- Immunisations should not be given before the scheduled age unless there is a clear indication.
The first dose of primary immunisations can be given from 6 weeks if there is a clear indication, e.g. travel to an endemic country.
Administration of the first set of primary immunisations before 6 weeks is not recommended as it could result in a sub-optimal response.
MMR can be given from 6 months, e.g. during a local outbreak or travel to endemic country. Any dose of MMR given before age 1 year should be discounted, and two further doses of MMR will be required at the appropriate ages.
Delaying primary infant immunisations beyond 8 weeks leaves babies unprotected against serious infections such as whooping cough, which can be very severe in the very young. It is not necessary to wait for the 6–8 week examination before giving the 8 week primary immunisations.
Evert effort should be made to ensure that all children and adults are immunised, even if they are older than the scheduled age: no opportunity to vaccinate should be missed.
The exception is rotavirus, where vaccination after the recommended age is more likely to result in intussception. Rotavirus vaccine should not be started if the infant is aged 15 weeks or older, and not given at all if aged 24 weeks or older.
CHILDHOOD IMMUNISATION PROGRAMME
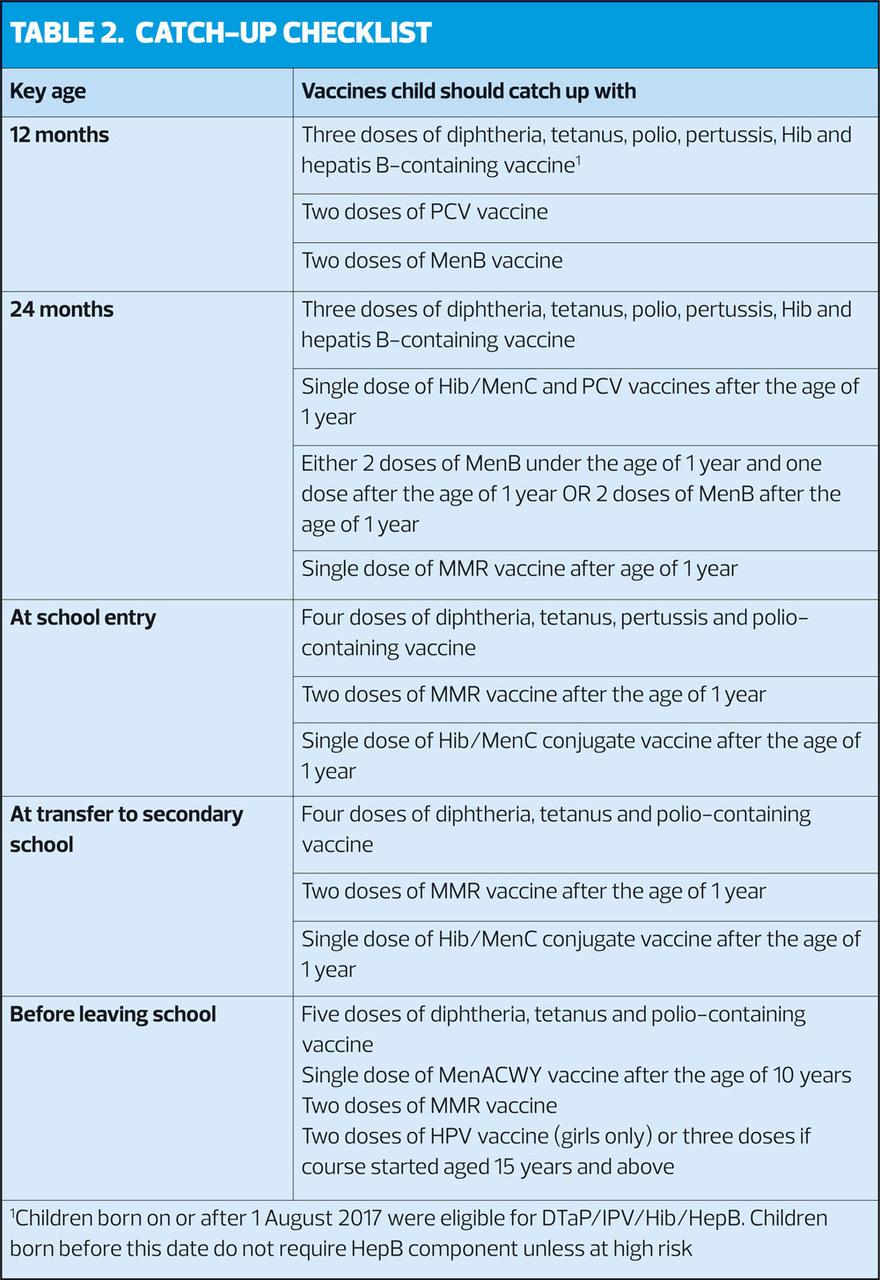
When children attend for any vaccination, check that they are up-to-date with any vaccinations that they should have received earlier (Table 2). Catch-up doses should be administered as soon as possible, but recommended intervals between doses should be observed.
PREMATURE INFANTS
Premature infants should have their immunisations at the appropriate chronological age, counted from their date of birth. Vaccinations should not be delayed or withheld. There is, however, an increased occurrence of apnoea in infants who were born very prematurely (≤ 28 weeks gestation) who are vaccinated in hospital should have respiratory monitoring for 48-72 hours when given the first dose, and if the child has apnoea, bradycardia or desaturations after the first immunisation, the second should also be given in hospital so they can have respiratory monitoring.
ADULT IMMUNISATION PROGRAMME
- MMR should be offered to all young adults who have not had two doses
- Check MMR status of all women of child bearing age, who should be offered MMR to prevent rubella in pregnancy
- Offer MenACWY vaccine to all individuals up to age 25 years who have never received a MenC-containing vaccine
- Older adults (65 years and older) should be offered a single dose of pneumococcal polysaccharide vaccine if they have not previously had it,
- Annual flu immunisation should be offered from age 65 years
- Adults aged 70 and over are eligible for shingles vaccination until their 80th birthday
UNKNOWN OR INCOMPLETE IMMUNISATION STATUS
Some patients will present having nor received some or all of their immunisations, or may have an unknown immunisation history. If born in the UK, make every effort to clarify what immunisations they may have had. If they have not completed the routine immunisation programme appropriate for their age should have the doses they have missed.
If the patient (adult or child) has come to the UK without a documented or reliable verbal history of immunisation, they should be assumed to be unimmunised and a full course of immunisations should be planned. Be aware that people from areas of conflict or groups that may have been marginalised in their country of origin e.g. refugees, gypsy or other nomadic travellers) may not have had good access to immunisation services.
An algorithm for vaccination individuals with uncertain or incomplete immunisation status is available at: https://www.gov.uk/government/publications/vaccination-of-individuals-with-uncertain-or-incomplete-immunisation-status
The immunisations offered in other parts of the world may differ from those in the UK programme – see http://apps.who.int/immunization_monitoring/globalsummary for details. Routine pre-school and subsequent boosters should be given according to the UK schedule.
VACCINATIONS FOR PREGNANT WOMEN
All pregnant women should be offered:
- Flu vaccination (inactivated influenza vaccine)
- Pertussis vaccination (dTaP/IPV) between weeks 16 and 32 of each pregnancy (probably best offered after the fetal anomaly scan at around 20 weeks)
Routine testing for rubella susceptibility ceased in 2016. Pregnant women should have vaccine status checked during or after pregnancy and offered any outstanding doses of MMR soon after delivery. MMR should not be given during pregnancy.
INTERVALS BETWEEN VACCINES
Doses of inactivated vaccines can be administered at any time before, after or at the same time as other inactivated or live vaccines.
A minimum of 4 weeks is recommended between successive doses of the same vaccine (8 weeks for some vaccines, e.g. PCV). Early vaccination should be avoided unless necessary to ensure rapid protection or to improve compliance, and additional doses may be recommended to ensure longer term protection.
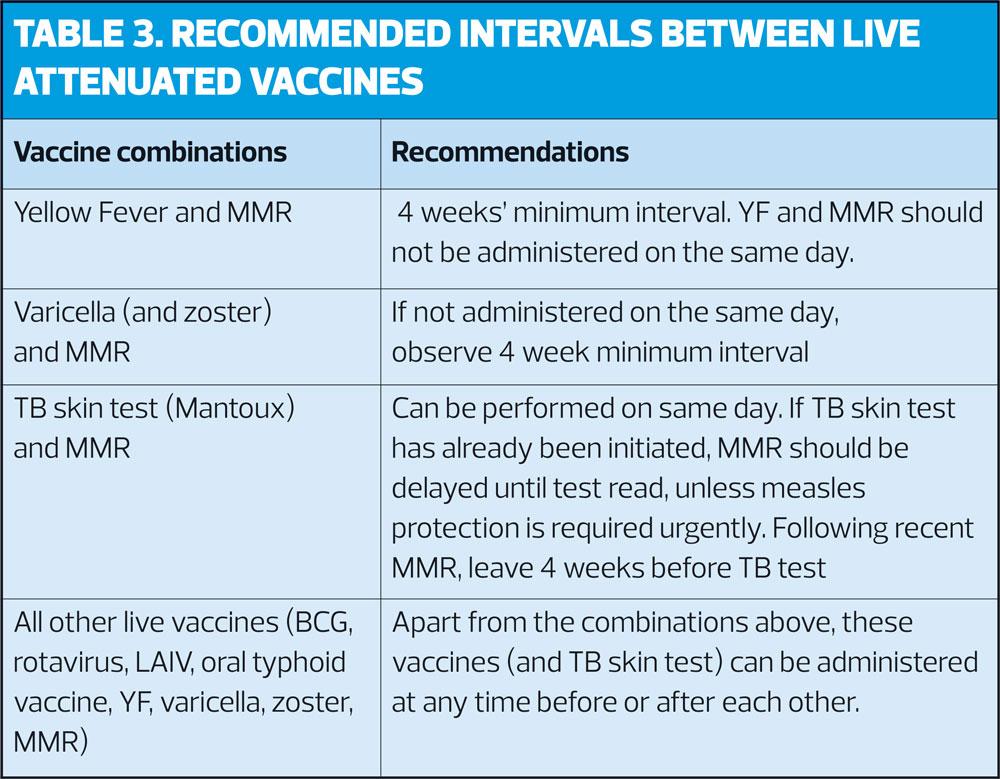
Intervals for administration of more than one live attenuated vaccine are shown in Table 3.
Reference
The Green Book Chapter 11: The UK immunisation schedule, April 2019.
Related guidelines
View all Guidelines