FSRH Clinical Guideline: Overweight, Obesity and Contraception
FSRH July, 2019
FSRH July, 2019
The Faculty of Sexual and Reproductive Healthcare (FSRH) has issued new guidelines to clarify safe and effective contraceptive options for women who are overweight or obese. The new guidance reviews the impact of weight on contraceptive effectiveness and safety as well as the effect of contraceptive use on weight. It also considers contraceptive use in relation to medical and surgical interventions for weight loss.
Currently it is estimated that over half of the women in the UK are overweight or obese, and obesity rate continue to rise among women of reproductive age. Among women who are obese, 5.3% will become pregnant each year.
While women with obesity may experience reduced fertility, the majority will continue to ovulate on a regular basis and should consider themselves as having the potential to become pregnant. And contrary to common misconceptions, the weight of adult women is not associated with reduced sexual activity.
Safe and effective pregnancy prevention and planning is critical as women with obesity, particularly those with comorbidities, are at significantly higher risk of complications in pregnancy and childbirth. These risks include gestational hypertension, diabetes, pre-eclampsia, caesarean delivery and postpartum haemorrhage. The unborn baby is at higher risk of complications such as growth restriction, macrosomia (significantly larger than average fetus), neural tube defects and stillbirth.
Obesity is associated with increased risk of cardiovascular disease and metabolic disorders, and these are important considerations when selecting contraceptive methods. The FSRH says it is a ‘clinical and public health necessity’ for healthcare professionals to understand the effectiveness and safety of contraception in women with raised body mass index (BMI) to assist them in making informed choices.
The guidelines provide recommendations for each contraceptive method, based on the following criteria:
- Does raised BMI affect contraceptive effectiveness?
- Does raised BMI affect safety?
- Does the method lead to weight gain?
- Are there non-contraceptive health benefits in women with raised BMI?
Effectiveness
Contraceptive effectiveness is determined by several factors including the user’s adherence and sexual behaviour, as well as the inherent efficacy of the method. Methods that require consistent and correct use are generally less effective than those requiring little or no user involvement, such as long-acting reversible contraception (LARC) or sterilisation.
Safety considerations
Women who are obese are at increased risk for other diseases and health conditions, and these risks need to be considered when providing contraception. Relevant comorbidities include venous thromboembolism (VTE), hypertension, dyslipidaemias, type 2 diabetes, CVD, stroke and some cancers (i.e. endometrial or breast cancer)
Weight gain
Weight gain is an important concern for many reasons, and particularly so for those with raised BMI. Perceived weight gain is a common reason for discontinuation of the oral contraceptive pill, for example, but women’s perceptions of weight gain do not always reflect their actual weight. Most studies into contraception and impact on weight gain are based on populations of women no more than 130% of ideal body weight, and few or no obese women are included.
Health benefits
The main benefit of contraceptive use is the prevention of pregnancy. Unplanned pregnancy in an obese woman carries greater risks than in women with normal BMI. There is little evidence specifically for other benefits in women who are overweight.
RECOMMENDATIONS
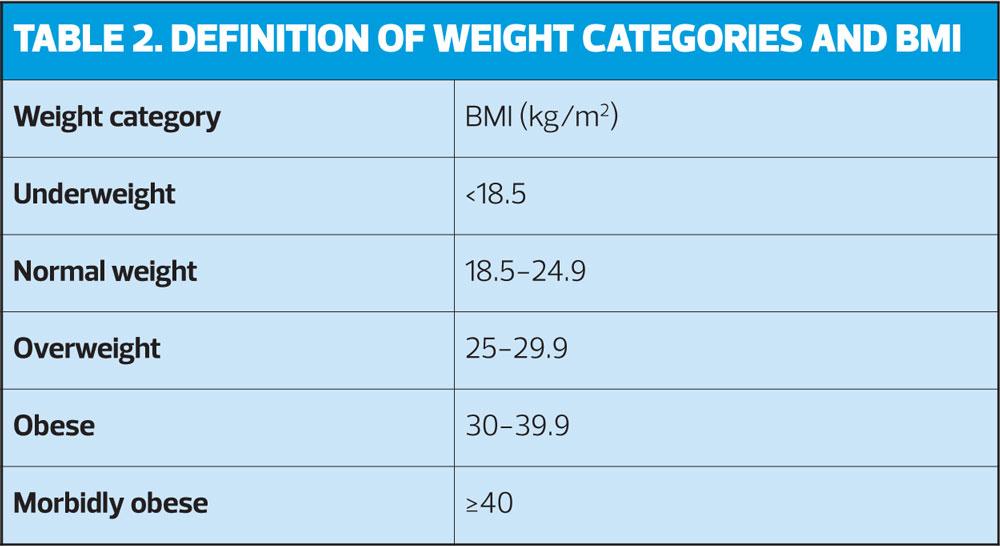
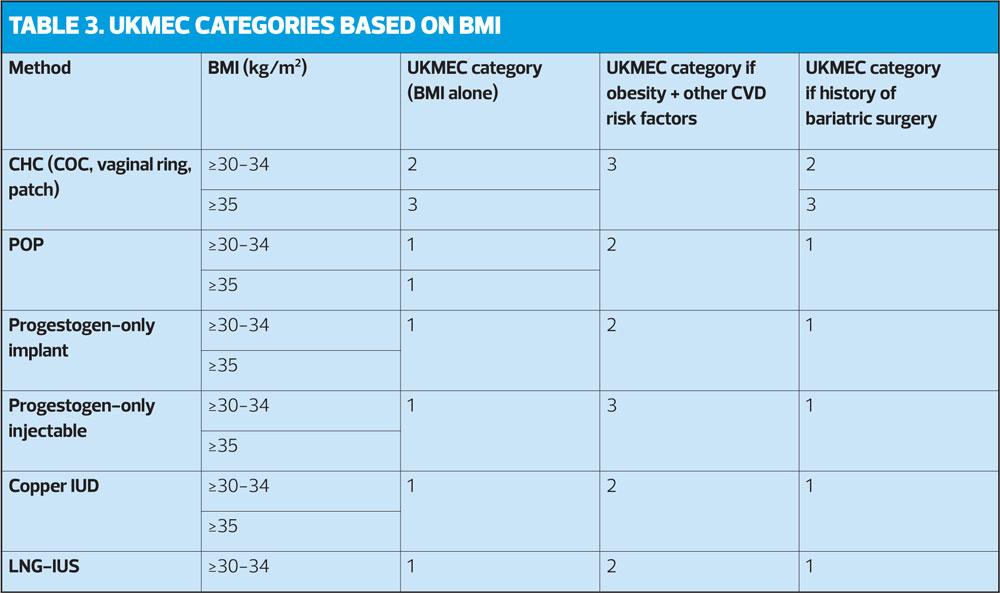
- For women with a BMI 35kg/m2 or greater, the potential risks associated with use of combined hormonal contraception (CHC) generally outweigh the benefits.
- Intrauterine contraception (IUC) is a highly effective method of contraception and its effectiveness is not affected by body weight or BMI. The available evidence suggests that IUC is a safe contraceptive option for women who are overweight or obese.
- Past concerns that women with raised weight or BMI would require a double dose of progestogen-only pills, early DMPA injections or early replacement of the contraceptive implant are refuted; the evidence indicates that the effectiveness of these methods is not affected by body weight or BMI.
- When providing contraception to women with raised BMI, healthcare professionals should – with the woman’s consent – raise the subject of weight, enquire whether BMI is of concern and signpost the woman to appropriate weight management support if she wants it.
Combined hormonal contraception (CHC)
- Most evidence suggests that effectiveness of combined oral contraception (COC) is not affected by body weight or BMI, but there is limited evidence to suggest possible reduced efficacy of the patch in women weighing ≥90kg. The effectiveness of the vaginal ring is not effected by body weight or BMI (limited evidence).
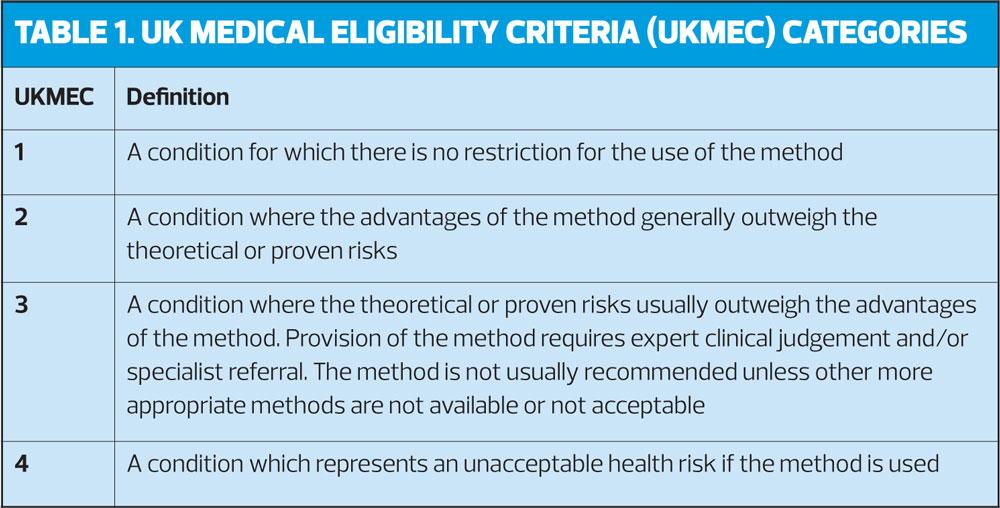
- CHC use is UKMEC 2 in women with BMI ≥30-34kg/m2 and UKMEC 3 for women with BMI ≥35kg/m2.
Inform women with obesity that:
- Risk of thrombosis increases with increasing BMI
- Current CHD use is associated with an increased risk of VTE, and small increased risk of myocardial infarction and stroke
- If BMI is ≥35kg/m2 the risks of CHC generally outweigh the benefits
Progestogen-only implants
- The etonogestrel (ENG) implant is a highly effective method of contraception; efficacy is not affected by body weight or BMI. The available evidence suggests that ENG is safe in women who are overweight or obese. The licensed duration of the ENG implant is 3 years, and is the same for women of all weight categories.
Progestogen-only injectable (DMPA)
- The available evidence suggests that effectiveness if DMPA injectable contraction is not affected by body weight or BMI. However, it is not possible to confirm or exclude the possible link between DMPA use and VTE.
- While obesity alone does not restrict DMPA use (UKMEC 1), when obesity is one of multiple risk factors for CVD (e.g. smoking, diabetes, hypertension) it becomes UKMEC 3.
- DMPA use is associated with some weight gain, particularly in women
- If using intramuscular DMPA consider using a longer-length needle or deltoid administration to ensure it reaches the muscle layer OR consider using subcutaneous DMPA.
Progestogen-only pill (POP)
- The available evidence suggests that effectiveness of POP is not affected by body weight or BMI, and that is a safe option for women who are overweight or obese.
- Double-dose POP is not recommended for women who are overweight or obese.
Emergency contraception (EC)
- Levonorgestrel 1.5mg EC (LNG-EC) appears to be less effective for women with BMI >26kg/m2 or weight >70kg.
- Ulipristal acetate EC (UPA-EC) may be less effective in women with BMI >30kg.m2 or weight >85kg.
- In women with BMI >26kg/m2 or weight >70kg, consider UPA-EC, or if this is not suitable, double-dose (3mg) LNG-EC. The effectiveness of double-dose LNG-EC is not known. Double-dose UPA-EC is not recommended for women of any body weight or BMI.
- The effectiveness of the copper intrauterine device is not affected by body weight or BMI: this is the most effective method of emergency contraception
- Women should be advised that the effectiveness of oral contraception, including oral EC, could be reduced during use of weight loss medications or after bariatric surgery. Where possible, these should be avoided in favour of non-oral methods.
FSRH Clinical Guideline: Overweight, Obesity and Contraception