Emergency contraception, FSRH 2017
This emergency contraception guideline gives clear evidence-based information to UK healthcare professionals, nurses, doctors, pharmacists and all those involved in advising and caring for women who wish access emergency contraception, and places more emphasis on the role of the copper IUD in emergency contraception.
Reproduced with kind permission of FSRH. The full text of the guideline is available at https://www.fsrh.org/Public/Documents/ceu-clinical-guidance-emergency-contraception-march-2017.aspx
The 2017 Emergency contraception guideline gives clear evidence-based information to UK healthcare professionals, nurses, doctors, pharmacists and all those involved in advising and caring for women who wish access emergency contraception.
In line with NICE guidance, recommendations mark a new emphasis on healthcare professionals advising women that the copper IUD, is the most effective method of emergency contraception and that it provides extremely effective long-term contraception.
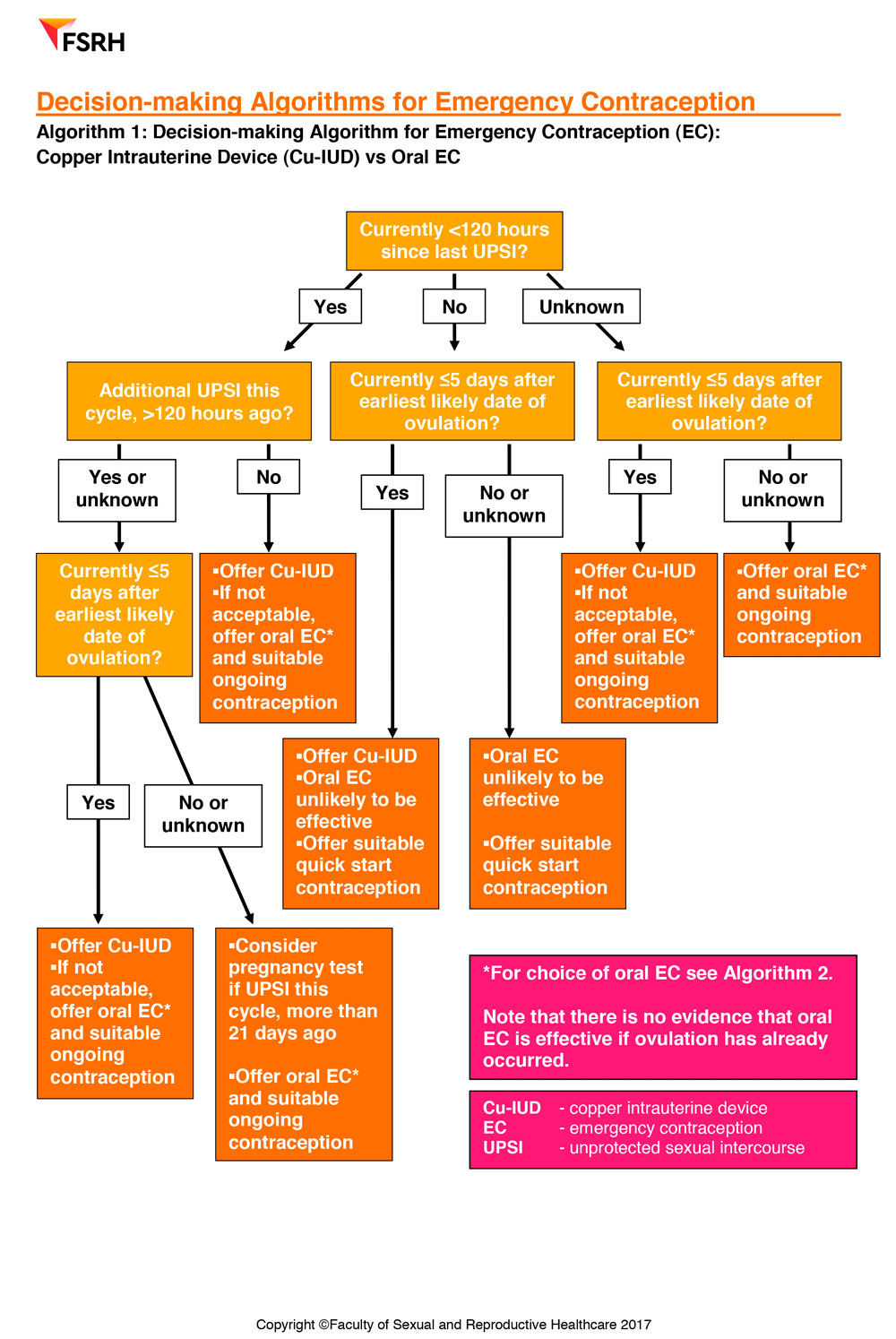
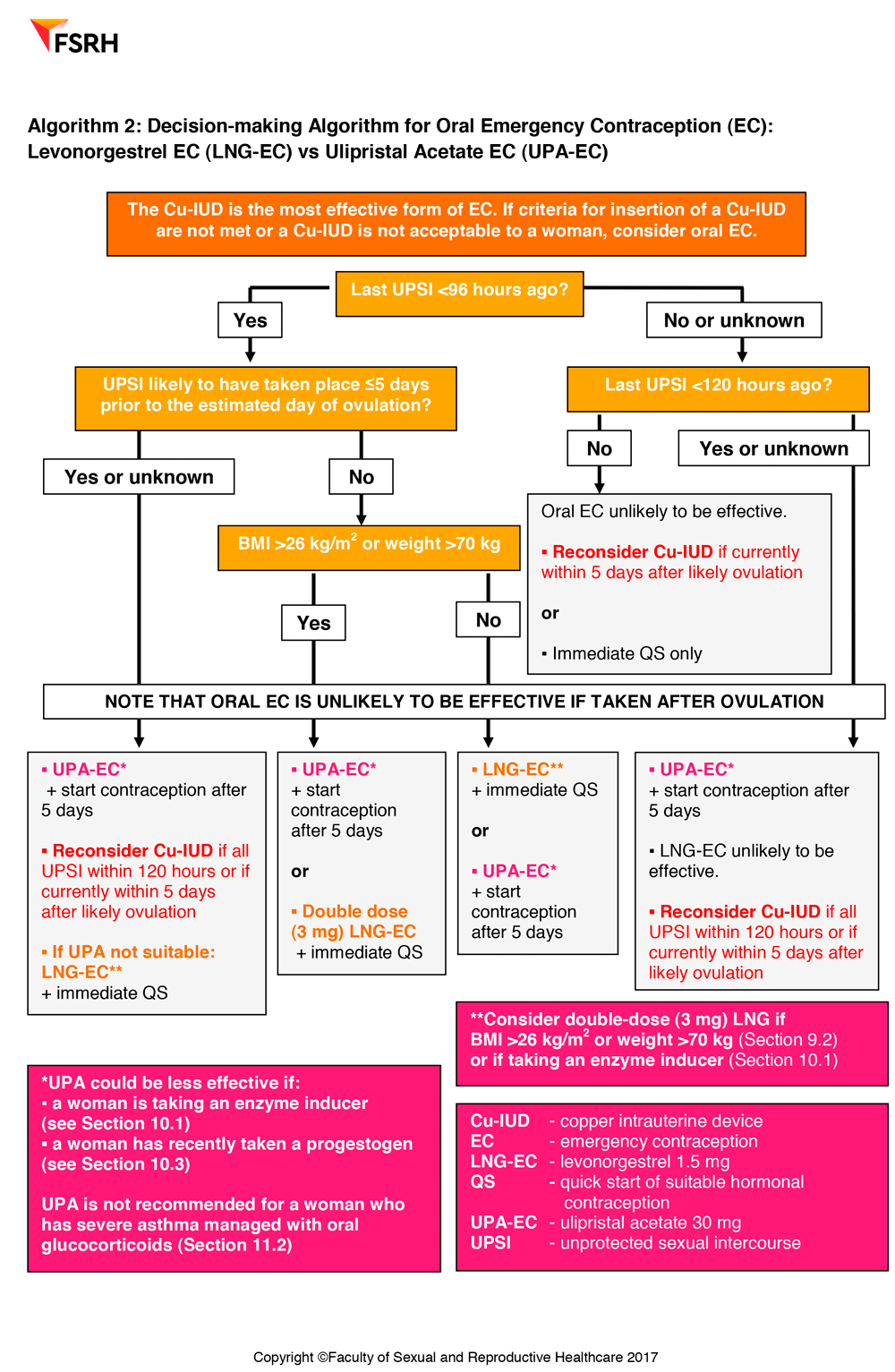
Emergency Contraception also includes practical decision making guides to help healthcare professionals in their decision making around using the copper intrauterine device or oral emergency contraception and decision making around the use of oral emergency contraception – levonorgestrel (LNG-EC) or ulipristal acetate.
RECOMMENDATIONS
INDICATIONS
Emergency contraception (EC) is indicated for women who do not wish to conceive after unprotected sexual intercourse (UPSI) that has take place on any day of a natural menstrual cycle.
Women who do not wish to conceive should also be offered EC:
- If their regular contraception has been compromised or has been used incorrectly
- From Day 21 after childbirth unless the criteria for lactational amenorrhea are met
- From Day 5 after abortion, miscarriage, ectopic pregnancy or uterine evacuation for gestational trophoblastic disease (GTD).
PROVISION
EC providers who cannot offer all EC methods should give women information about the other methods and point them to services that can provide them.
Providers of oral EC should advise women that oral EC methods do not provide contraceptive cover for subsequent UPSI and that they will need to use contraception or abstain from sex to avoid further risk of pregnancy.
Women requesting EC should be given information on all methods of ongoing contraception and how to access them.
EFFECTIVENESS
The Cu-IUD is the most effective method of EC
Ulipristal acetate EC (UPA-EC) is effective for EC up to 120 hours after UPSI
Levonorgestrel EC (LNG-EC) is licensed for up to 72 hours after UPSI and is ineffective if taken more than 96 hours after UPSI
UPA-EC has been shown to be more effective than LNG-EC
Available evidence suggests that oral EC administered after ovulation is ineffective
BMI
The effectiveness of the Cu-IUD is not affected by weight or BMI.
Higher weight or BMI may reduce the effectiveness of oral EC, particularly LNG-EC
DRUG INTERACTIONS
Enzyme-inducing drugs may reduce the effectiveness of oral EC. A 3mg dose of LNG can be considered, but the effectiveness of this dose is not known. A double dose of UPA-EC is NOT recommended.
Women using enzyme-inducing drugs should be offered a Cu-IUD if appropriate.
Effectiveness may be reduced if a woman takes progestogen in the 5 days after or 7 days before taking UPA-EC
CONTRAINDICATIONS
Contraindications to insertion of a Cu-IUD for EC are the same as those for routine IUD insertion
UPA-EC is not suitable for women who have severe asthma controlled by oral glucocorticoids
BREASTFEEDING
Breastfeeding women should be advised not to breastfeed, and to express and discard milk for a week after they have taken UPA-EC
Limited available evidence indicates that LNG-EC has no adverse effects on breastfed infants
METHODS
All women requiring EC should be offered a Cu-IUD if appropriate as it is the most effective method.
A Cu-IUD can be inserted up to 5 days after the first UPSI in a natural menstrual cycle, or up to 5 days after the earliest likely date of ovulation (whichever is later).
If a Cu-IUD is inappropriate or unacceptable, oral EC should be taken as soon as possible if there has been UPSI within the last 5 days
UPA-EC should be considered as first-line oral EC for a woman who has had UPSI 96-120 hours ago (even if she also had UPSI within the last 96 hours) and for women who have had UPSI within the last 5 days if this is likely to be during the 5 days before the estimated date of ovulation.
Oral EC administered after ovulation is ineffective.
All methods of EC, including the Cu-IUD, should be offered to adolescents who need EC and to women requiring EC after sexual assault.
ORAL EC USE
UPA-EC or LNG-EC can be used if the woman has had UPSI earlier in the same cycle as well as within the last 5 days as they do not disrupt an existing pregnancy and are not associated with fetal abnormality.
If a woman has already taken UPA-EC once or more in a cycle, she can use UPA-EC again after further UPSI in the same cycle.
If a woman has already taken LNG-EC once or more in a cycle, she can use LNG-EC again after further UPSI in the same cycle.
If a woman has already taken UPA-EC, she should not take LMG-EC in the following 5 days.
If a woman has already taken LNG-EC, UPA-EC taken in the following 7 days may be less effective.
FUTURE CONTRACEPTION
Cu-IUD provides effective ongoing contraception.
Oral ED does not provide ongoing contraception.
After oral EC there is a pregnancy risk If further UPSI and ovulation occurs later in the same cycle.
After LNG-EC, women can start suitable hormonal contraception immediately. They should use condoms or abstain from sex until contraception becomes effective.
After UPA-EC, women should wait 5 days before starting suitable hormonal contraception. They should use condoms or abstain from sex during those 5 days and until contraception becomes effective.
FSRH. Emergency Contraception; 2017, updated 2023. https://www.fsrh.org/Public/Documents/ceu-clinical-guidance-emergency-contraception-march-2017.aspx