Travel update September 2025
Jane Chiodini, MSc(Travel Med), RGN, FFTM RCPS(Glasg), QN, Director of Education, Faculty of Travel ...
Practice Nurse 2025;55(5):31
Jane Chiodini brings us up to date with the travel-associated infections report from UKHSA and the latest developments on the chikungunya virus (CHIKV) vaccine
CHIKUNGUNYA AND OROPOUCHE
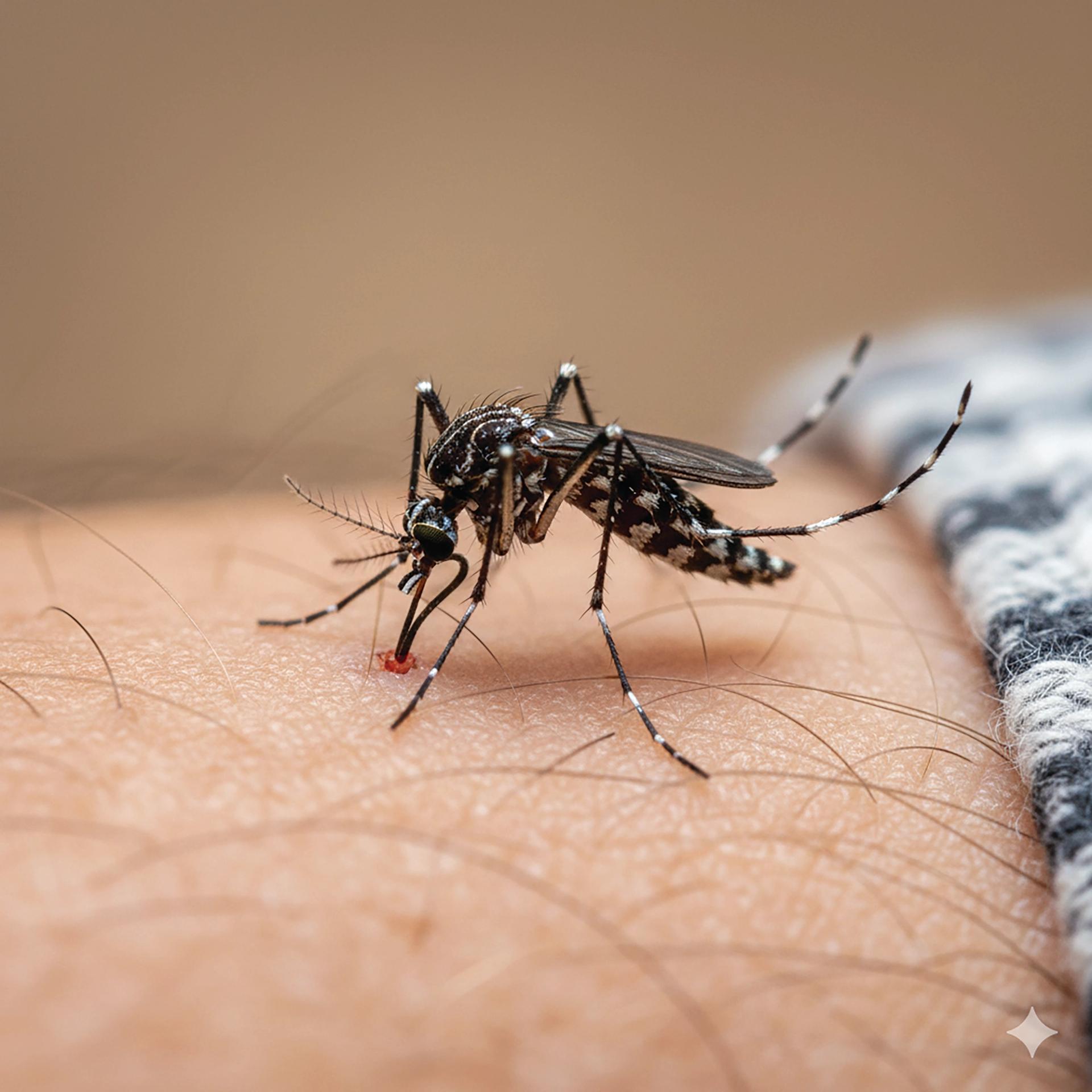
The latest UKHSA publication of travel-associated infections in England, Wales and Northern Ireland, published on 14 August, reported that the number of imported chikungunya cases increased to 73 in the six-month period from January to June 2025, up from 27 during the same timeframe in 2024. Most cases were reported following travel to Sri Lanka, India and Mauritius, aligning with ongoing local outbreaks in countries in the Indian Ocean region.There were also three cases of Oropouche virus in travellers returning to the UK. This is the first time the UK has reported Oropouche virus cases, with all cases associated with travel to Brazil. Dengue cases were lower at 161, down from 490 during the same period in 2024, with Thailand, Brazil and Indonesia as the most frequently reported travel destinations. More details can be found at https://www.gov.uk/government/publications/travel-associated-infections/travel-associated-infections-in-england-wales-and-northern-ireland-january-to-june-2025. It therefore remains very important to discuss mosquito bite avoidance with travellers as one of the measures to combat these diseases if they travel to ‘at risk destinations’.Knowledge of the dengue and chikungunya vaccines is also now necessary, which I wrote about in the last issue (See Practice Nurse May-June 2025;55(4):31).Since that time, the JCVI has published guidance on the use of the Chikungunya vaccines at https://www.gov.uk/government/publications/chikungunya-vaccine-for-uk-travellers-jcvi-advice-16-july-2025/chikungunya-vaccine-in-uk-travellers-jcvi-advice but publication of the Green Book chapter is still awaited.
Development on guidance of use of Ixchiq, which is the live chikungunya vaccine, took a dramatic turn in the USA on 25 August when the US Food and Drug Administration (FDA), the equivalent of the MHRA in the UK, announced the suspension of the vaccine license, due to serous safety concerns.However, in an updated news item on 29 August from NaTHNaC on TravelHealthPro, advice on vaccine use in the UK has not altered – but it would be wise to keep watch for updates at https://travelhealthpro.org.uk/news/843/chikungunya-vaccination-information. The other chikungunya vaccine, Vimkunya is being marketed for use in the UK and should be available in private travel clinics.The country recommendations for CHIKV are now also available on the country specific pages of TravelHealthPro.
TRAVAX and FOR FOR TRAVEL
These travel services from Public Health Scotland (PHS) turned 40 years old this year. I began using them back in 1995, when I started studying travel medicine. The Travel and International Health Team have always been very innovative and over the years, provided significant help to healthcare professionals providing a travel health service through TRAVAX and for the general public on Fit for Travel. It was my pleasure to present at the TRAVAX 30th birthday meeting in 2015 on ‘21st Century Travel Health Communication’ so for fun I’ve uploaded it onto a page on my website alongside some of the other presentations I’ve given over the years. https://www.janechiodini.co.uk/about/presentations/
On 22nd September 2025, due to resource issues, the decision was taken to retire both websites. Healthcare professionals and the public alike are advised to go to https://travelhealthpro.org.uk/ for all future travel health advice. I would like to personally thank all those involved in these service spanning the forty years. They were truly at the forefront of putting travel medicine firmly on the map in the UK and the websites will be missed and fondly remembered.
VACCINATOR COMPETENCY ASSESSMENT
My last travel health update discussed the newly published National minimum standards and core curriculum for vaccination training in relation to travel health. Appendix A, the Vaccinator competency assessment tool workbook to accompany this new guidance is now available to download at https://assets.publishing.service.gov.uk/media/688a481f1affbf4bedb7b0f1/UKHSA_Appendix_A_Vaccinator_competency_assessment_tool_workbook.pdf As a PDF, it appears it would need to be printed out and completed manually, unless you have a recent version of Adobe Acrobat which enables you to complete forms online.
For news of further educational opportunities, including free monthly webinars from the Faculty of Travel Medicine until the end of the year, see the updated events page on my website at https://www.janechiodini.co.uk/links/events/
Related articles
View all Articles