Travel health update
Jane Chiodini MSc (Travel Med), RGN, RM, FFTM RCPS(Glasg)
Jane Chiodini MSc (Travel Med), RGN, RM, FFTM RCPS(Glasg)
New antibiotic for travellers diarrhoea; availability of hepatitis A and typhoid vaccines; nurses' role in travel health - study
New antibiotic for travellers' diarrhoea
It is estimated that 20 - 60 % of travellers will suffer travellers' diarrhoea (TD), which is especially common in those travelling from a resource-wealthy to resource-poor destination. This non vaccine preventable infection, caused by parasites, bacteria and viruses is usually contracted through the consumption of contaminated food and water. TD is described as three or more unformed stools in a 24 hour period. Prevention is by observing food, water and personal hygiene advice but evidence shows that such measures are poorly adhered to. The mainstay of treatment is to ensure adequate hydration is maintained, which is vitally important for young children, the elderly and those with pre-existing medical conditions. Anti-motility drugs can be used to make the symptoms more manageable, however caution must be applied in their use. Antibiotic treatment (commonly fluroquinolones) can both shorten and reduce the severity of the illness. An antibiotic licensed specifically for the treatment of TD has recently been launched in the UK. Rifaxamin (Xifaxanta(R)) can be prescribed for uncomplicated, non-invasive TD in adults. This oral antibiotic remains very active in the gastrointestinal tract with minimal systemic absorption (less than 1%). A dose of 200mgs three times a day for three days is advised and travellers could opt to travel with a course for standby treatment. For further details see the Summary of Product Characteristics at www.medicines.org.uk. Norgine, the manufacturers of rifaxamin, have also developed a useful patient website about TD at www.travellersdiarrhoea.co.uk
Vaccine shortages - hepatitis A and typhoid
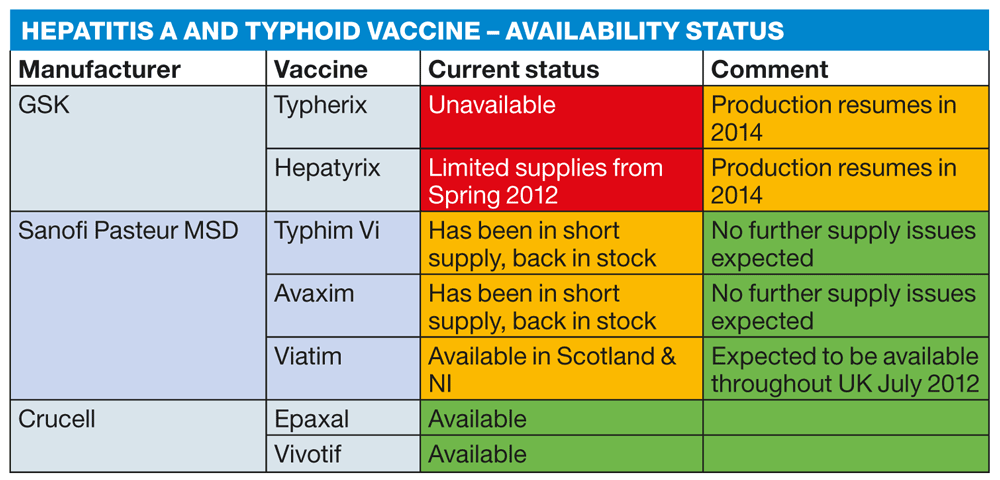
Discontinuation of the production of the typhoid vaccine until 2014 from GlaxoSmithKline (GSK) is taking its effect on many practices, similarly with combination hepatitis A and typhoid vaccines. The GSK product Hepatyrix is also currently unavailable. However, limited supplies should be back in stock in the Spring but once exhausted, like Typherix, Hepatyrix will then not be available until 2014. The alternative injectable typhoid vaccine, Typhim Vi, from Sanofi Pasteur MSD has also been in short supply, but I'm now informed by their medical department that both Typhim Vi and Avaxim are fully back and they are not expecting to have any further supply issues with these products. Their combined hepatitis A and typhoid vaccine, Viatim, is currently available in Scotland and N. Ireland and is due to be back in full supply in the rest of the UK during July. Crucell manufacture the alternative hepatitis A vaccine, Epaxal, and also the oral typhoid vaccine called Vivotif. Vivotif is available on the NHS and full details about its administration and use can be found at www.dh.gov.uk/greenbook
Survey needs your support!
An international research project is underway looking at the nurse's role in the provision of travel health care in the UK, Australia and Japan. The researchers comprise three leading nurses in travel medicine from these destinations. The work hopes to compare, contrast and describe the competencies, educational and professional needs of, and opportunities for, registered nurses working in travel health. The survey will only be successful if sufficient nurses participate in the anonymous online questionnaire at https://www.surverymonkey.com/s/B639DGC Having undertaken the survey, I can confirm if only takes a few minutes to complete and is an exciting piece of work so please participate if you can, the deadline is 2 April 2012.