The respiratory year in review
Beverley Bostock-Cox, RGN, MSc, QN, Nurse Practitioner, Mann Cottage Surgery Moreton in Marsh, Clini...
Beverley Bostock-Cox, RGN, MSc, QN, Nurse Practitioner, Mann Cottage Surgery Moreton in Marsh, Clinical Lead Education for Health Warwick
As another year comes to an end we take the opportunity to look back at the year from a respiratory perspective and see what has been happening over the past 12 months and to muse a little over where 2016 may take us
At the end of 2015 we are, in some respects, in limbo as we await updated guidance from NICE on asthma and chronic obstructive pulmonary disease (COPD). But while we wait research continues and new therapies, approaches, ideas and concerns continue to evolve, offering the potential to improve the care offered to patients. In this article we also consider some of the changes that have affected general practice nurses (GPNs) this year and how we can ensure that we are suitably prepared to meet the new challenges that the next year will bring.
ASTHMA
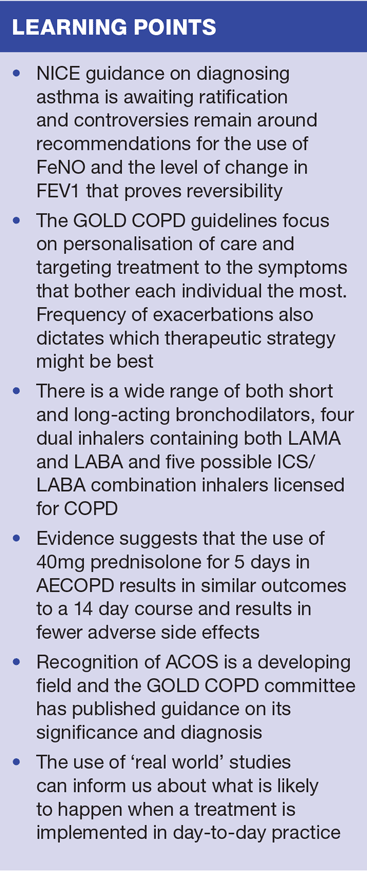
NICE guidance on diagnosing asthma was due to be published this year, but at the time of writing this guidance is still awaiting ratification.1 The guidance was deemed necessary because it was thought that too many people with a diagnosis of asthma had had the diagnosis made without recourse to objective measurements of reversible, variable obstructive lung disease. The National Review of Asthma Deaths (NRAD) suggested that diagnostic processes were not always followed for the original diagnosis or for assessment of exacerbations.2 As a result, NICE decided to focus on how the diagnosis should (or could) be made.
The BTS/SIGN asthma guidelines include advice on making the diagnosis, stressing the key importance of history taking.3 These guidelines also include much on managing the condition, both when stable and during exacerbations. The proposed NICE guidance looks specifically at diagnosis and includes a more detailed overview of how to use objective tests to ensure that the diagnosis is made with a high degree of accuracy. In view of the fact that it has not yet been finalised or approved, it would be inappropriate to go into the detail of what has been suggested or ponder too much on what might end up in the final guidelines, but areas which have generated some discussion in the respiratory world already will be considered here.
Fractionated exhaled nitic oxide
Fractionated exhaled nitric oxide (FeNO) testing offers the opportunity to measure breath levels of nitric oxide – a marker of asthma-related inflammation – to identify people with asthma where there is diagnostic doubt. It can also be used in people who are poorly controlled to identify those individuals where inflammation is inadequately controlled.4
NICE has recommended that FeNO testing could be used in these situations, but the cost of the equipment, and in particular the single patient use mouthpieces, meant that many in primary care said this was unworkable. The NICE diagnostic guidance document on FeNO states that this test can be used in combination with clinical examination and other investigations to help confirm a diagnosis in adults and children where there is an ‘intermediate’ probability of asthma, based on the BTS/SIGN criteria.5 The guidance also recommends FeNO as an option to support decisions about asthma treatment in people who remain symptomatic despite using inhaled corticosteroids.
Reversibility
Another proposal from NICE was that the suggested level for proof of reversibility, currently set at a change in FEV1 of 400ml, should be decreased to 200ml. Some felt that this return to the lower level of change in FEV1, which had previously been advised in older guidelines, was a retrograde step since unequivocal evidence of reversibility should be required for an asthma diagnosis. However, the rationale for this proposal seems to be that if there is ANY evidence of reversibility it should be treated (with a low dose inhaled corticosteroid [ICS]) before other symptoms are treated. This gives a nod to the new respiratory kid on the block – asthma and COPD overlap syndrome (ACOS) – of which more later.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
It is clear that the NICE guidance on COPD is woefully outdated and out of kilter with current evidence, both at a national and international level. The Quality Standards encourage clinicians to make sure they get the diagnosis right before moving on to treating people appropriately.6 The focus remains on spirometry as the key objective diagnostic tool (in asthma as well as in COPD) and the Quality Standards reinforce the importance of the diagnosis being made using quality assured diagnostic spirometry (QADS), as laid out in the QADS document from Primary Care Commissioning a full two years ago.7
In contrast to the rather elderly NICE guidance on diagnosing and managing COPD, the GOLD guidelines have had their annual update, with another due soon, and remain the ‘go-to’ guidance for many people working in respiratory care.8 They are not without their detractors, however, with a lack of scientific evidence being cited as the main reason for handling them with care. Interestingly, there is a sea swell of change happening with regard to the evidence used for guideline development. A move towards using ‘real world’ data is gaining support, something that is discussed in more detail below.
GOLD guidance focuses on personalisation of care by recognising the symptoms that bother each individual patient most and targeting treatment to these symptoms. Frequency of exacerbations also dictates which therapy area might be best. Lung function tests are considered to be less important when deciding on treatment options as they are known to have little correlation with the symptoms the patient suffers from.9
When it comes to deciding on treatment options, new products became available this year meaning that there is now a whole range of short and long-acting bronchodilators as well as four dual bronchodilators (long acting muscarinic antagonists (LAMAs) and long acting beta 2 agonists (LABAs) in one inhaler) in a range of different devices. For those who need combination therapies (ICS/LABA) there are now five possible options that are licensed for COPD.
Acute exacerbations of COPD
Acute exacerbations of COPD (AECOPD) have been in the news this year as clinicians try to take a more personalised approach to managing these too. Studies suggest that as few as 20% of all AECOPD have an infective cause. Even the isolation of bacteria in sputum samples may not be diagnostic since people with stable COPD often have high bacterial loads in the lungs.10 The blanket use of antibiotics is therefore being debated, particularly at a time when antibiotic resistance is highlighted as a major risk to global health. While sputum colour has been linked to the likelihood of an exacerbation being caused by a bacterial infection,11 there is also some suggestion that using point of contact testing for c-reactive protein (CRP) might help to identify those patients who require antibiotics for their AECOPD.12 Further research is ongoing into this area.
Another study that has led to some people changing their advice on steroid use in AECOPD is the REDUCE study.13 This study compared the use of 40mg prednisolone for 5 days with 14 days of treatment. It demonstrated that using 40mg for 5 days had the same outcome as a longer course of treatment but that the side effects, such as raised blood pressure and blood glucose, were fewer. In addition 56 people in the 5 day group had another exacerbation within six months compared to 57 in the 14 day group. REDUCE therefore provided some evidence for giving a shorter course of treatment and reviewing the patient at the end of the 5 days to assess response. Although the study was published in 2013, the information is now being included in rescue pack management plans in more and more clinical commissioning groups (CCGs) around the country.
Eosinophils
COPD is an area where much research is undertaken and there are likely to be further developments in the next 12 months. An area of developing interest is whether measuring eosinophils can help to identify those who are more likely to benefit from steroid-based therapies to treat COPD.14 Data presented at international meetings this year from studies such as this one by Pascoe and colleagues14 suggest, once again, that a more tailored approach to treating people with respiratory disease is moving ever closer.
ASTHMA AND COPD OVERLAP SYNDROME
The overlap between reversible and irreversible airways disease has long been recognised and many GPNs will be able to think of patients who have elements of both. In 2015, the term ACOS was increasingly used to describe this condition and ACOS was the subject of several presentations at national and international respiratory conferences. The GOLD committee has published their take on it,15 and it is likely that we’ll be hearing much more about it in the following months and years.
RCTs AND ‘REAL WORLD’ STUDIES
Although randomised controlled trials (RCTs) have always been considered to be the best evidence that can be used to inform clinical practice, more thought is being given these days to the fact that the very design of an RCT (tightly controlled and with carefully selected criteria for patient selection and exclusion) can mean that the findings cannot be applied to real life. ‘Real’ people have different characteristics – such as their ability to use an inhaler, their adherence to prescribed treatment and their lifestyles. These varying characteristics are often removed from an RCT to ensure that populations are as similar to each other as possible, making the trial population somewhat abnormal. A possibly better way of gathering information on which to base guidelines, then, could be to combine findings from RCTs with those that have come from studies which more closely resemble real life, so-called pragmatic or observational studies.
According to Sackett,16 the leading authority on evidence based medicine, taking this approach may enable us to see if the intervention works (via the RCT) and then tells us what is likely to happen when the treatment is implemented in day to day practice. Dr David Price, Professor of Primary Care Respiratory Medicine, has helped to set up the Respiratory Effectiveness Group (http://www.effectivenessevaluation.org/) whose aim, according to its website, is to recognise the potential value of real-life research and the importance of capturing real-life evidence to inform guidelines, drug licensing and prescribing decisions.
TECHNOLOGY AND LEARNING
One of the greatest changes in the dissemination of research over the past year is the use of technology and social media to allow people from around the globe to share information and learn together. Twitter activity explodes at conference time and nurses are sharing ideas and supporting one another through twitter chats and groups such as weGPNs.
It is very exciting to have instant and direct access to key individuals and organisations in 140 characters or less! At the recent Best Practice in Nursing conference it was quite a challenge to keep up with the torrent of tweets that were happening as a result of what was being said in real time at the conference. Technology is changing how we learn and eLearning is fast becoming the preferred method of education and professional development despite many people being a little wary of moving away from books and paper-based learning initially. Webinars have also become more popular allowing access to an expert in your surgery or living room, along with an opportunity to interact with them.
LINKING THE NMC CODE, THE 4 Ps AND RESPIRATORY CARE
Both the publication of the updated NMC code of conduct in March 2015,17 and the endorsement of the revalidation process in October 2015,18 offer a superb opportunity for GPNs involved in respiratory (or any other type) of care to review their current practice and reflect on how they are doing. The code is divided into four key areas, referred to as the ‘four Ps’:
1. Prioritise people
2. Practise effectively
3. Preserve safety
4. Promote professionalism and trust
In other words, the Code gives us a mandate to be the patient’s advocate by clearly stating that nurses (and midwives) should inspire trust and show professionalism by always putting patients first and ensuring that care is delivered safely and effectively.
Knowledge and understanding of respiratory care is ever changing and the range of therapy options can be bewildering. Working in practice can be stressful at times and it can be hard dealing with the sometimes competing interests of providing excellent patient care, dealing with resource restrictions and recognising the need to tick the right boxes, both literally and metaphorically. It can be a challenge to argue for longer appointment times or appropriate training and education to ensure that you always adhere to the four Ps. However, successful revalidation demands proper reflection in and on practice, a requirement to work through those reflections with another NMC registrant, and a need to prove that ongoing professional development has occurred. Rather than seeing this as a threat, these elements should be seen as a real opportunity to develop a thoughtful, intelligent and proactive body of practice nurses who will be able to offer, not only the very best of nursing care, but who will also help to wave a big flag for primary care nursing, attracting new nurses into surgeries and clinics around the UK.
CONCLUSION
In summary, it has been an interesting year for people delivering respiratory care and it seems likely that 2016 will bring even more food for thought with the final publication of the various guidelines mentioned above and a move towards real life evidence in practice. The challenge for general practice nurses, with our broad remit, is to keep up to date with all that is happening so that our patients get the benefit of us acting as advocates for them. We can do this by knowing how to recognise, diagnose, treat and offer ongoing management of their condition, whilst always keeping a focus on them as individuals with their own needs and priorities. Finally, we need to recognise that we need to support each other to meet the requirements of the new Code of conduct and the revalidation process. By doing this we can also help to improve patient care. Onward and upward!
REFERENCES
1. NICE. Asthma: diagnosis and monitoring of asthma in adults, children and young people draft guidance, 2015 https://www.nice.org.uk/guidance/gid-cgwave0640/resources/asthma-diagnosis-and-monitoring-draft-guideline2
2. Royal College of Physicians. National Review of Asthma Deaths: why asthma still kills, 2014. https://www.rcplondon.ac.uk/sites/default/files/why-asthma-still-kills-full-report.pdf
3. British Thoracic Society/Scottish Intercollegiate Guidelines Network (BTS/SIGN) Asthma guidelines, 2014. https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-asthma-guideline-2014/
4. Dweik RA, Boggs PB, Ezurum SP, et al. An Official ATS Clinical Practice Guideline: Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications, 2011 https://www.thoracic.org/statements/resources/allergy-asthma/feno-document.pdf
5. NICE. Measuring fractional exhaled nitric oxide concentration in asthma: NIOX MINO, NIOX VERO and NObreath, 2014 https://www.nice.org.uk/guidance/dg12/resources/measuring-fractional-exhaled-nitric-oxide-concentration-in-asthma-niox-mino-niox-vero-and-nobreath-1053626430661
6. NICE. COPD Quality Standard Update (draft for consultation), 2015 http://www.nice.org.uk/guidance/gid-qsd122/resources/chronic-obstructive-pulmonary-disease-qs-update-qs-draft-guidance-for-consultation2
7. Primary Care Commissioning. A guide to performing quality assured diagnostic spirometry, 2013 https://www.pcc-cic.org.uk/sites/default/files/articles/attachments/spirometry_e-guide_1-5-13_0.pdf
8. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Pocket guide to COPD diagnosis, management and prevention, 2015 http://www.goldcopd.org/uploads/users/files/GOLD_Pocket_2015_Feb18.pdf
9. Siafakas NM, Vermeire P, Pride NB, et al. Optimal assessment and management of chronic obstructive pulmonary disease (COPD) Eur Respir J 1995;8:1398–1420
10. Beasley V, Joshi PV, Singanayagam A, et al. Lung microbiology and exacerbations in COPD. Int J Chron Obstruct Pulmon Dis 2012;7:555–569
11. Allegra L, Blasi F, Diano P, et al. Sputum color as a marker of acute bacterial exacerbations of chronic obstructive pulmonary disease Res Med 2005;99(6):742-747
12. Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease JAMA 2013;309(22):2353-61
13. Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA 2013;309(21):2223-31.
14. Pascoe S, Locantore N, Dransfield NT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials The Lancet 2015;3(6) 435-442
15. GOLD. Diagnosis of Diseases of Chronic Airflow Limitation: Asthma COPD and Asthma-COPD Overlap Syndrome (ACOS), 2014 http://www.goldcopd.org/uploads/users/files/AsthmaCOPDOverlap.pdf
16. Sackett DL. Explanatory and pragmatic clinical trials Pol Arch Med Wewn 2011;121: 259-263
17. Nursing and Midwifery Council. Code of Conduct, 2015 http://www.nmc-uk.org/
18. Nursing and Midwifery Council. Revalidation, 2015 http://www.nmc.org.uk/standards/revalidation/
Related articles
View all Articles