What you need to know before prescribing controlled drugs
Stephanie Garner RGN BSc MSt (Cantab)
Stephanie Garner RGN BSc MSt (Cantab)
Association of Nurse Prescribers Advanced Nurse Practitioner, Wisbech
The long awaited and much campaigned for amendment to legislation regulating the supply of controlled drugs means that independent nurse prescribers can now provide the whole range of analgesia to their patients in pain. Our update provides an overview of the legal requirements of controlled drug prescribing
In April this year (2012) the Home Office published the statutory instrument that enables nurse and pharmacist independent prescribers to prescribe controlled drugs, authorises the mixing of controlled drugs and allows the use of patient group directions (PGDs) to supply morphine or diamorphine for immediate and/or necessary treatment.1 This amendment to the 1971 Misuse of Drugs Act allows patients to receive timely and appropriate medications from appropriately trained healthcare professionals. (Box 1).
With these changes coming into force it is pertinent for all nurse independent prescribers (or indeed those undertaking or thinking of undertaking the training to become an independent prescriber), to review the changes to controlled drug prescribing and the safe, effective and legal prescribing of them to ensure patients are not put at risk of harm.
This article aims to provide practice nurses with an overview of the Misuse of Drugs Regulations, controlled drug classes and schedules, the process for safe and effective prescribing of controlled drugs, the legalities of controlled drug prescription writing and the potential for abuse.
HISTORICAL ASPECTS
Misuse of Drugs Act (1971)
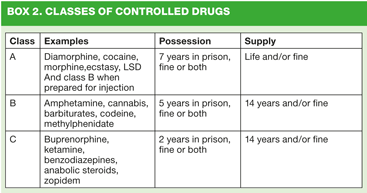
The Misuse of Drugs Act was introduced in 1971 to bring the UK in line with the Single Convention on Narcotic Drugs, the Convention on Psychotropic Substances, and the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances. The Act details the three classes of controlled drugs related to their level of harmfulness when they are misused, with Class A being the most harmful. It also states the differing penalties that can be enforced by the courts for illegal or unlicensed manufacture or possession, and possession with intent to supply, which vary from fines to life imprisonment(Box 2).
Misuse of Drugs regulations (2001)
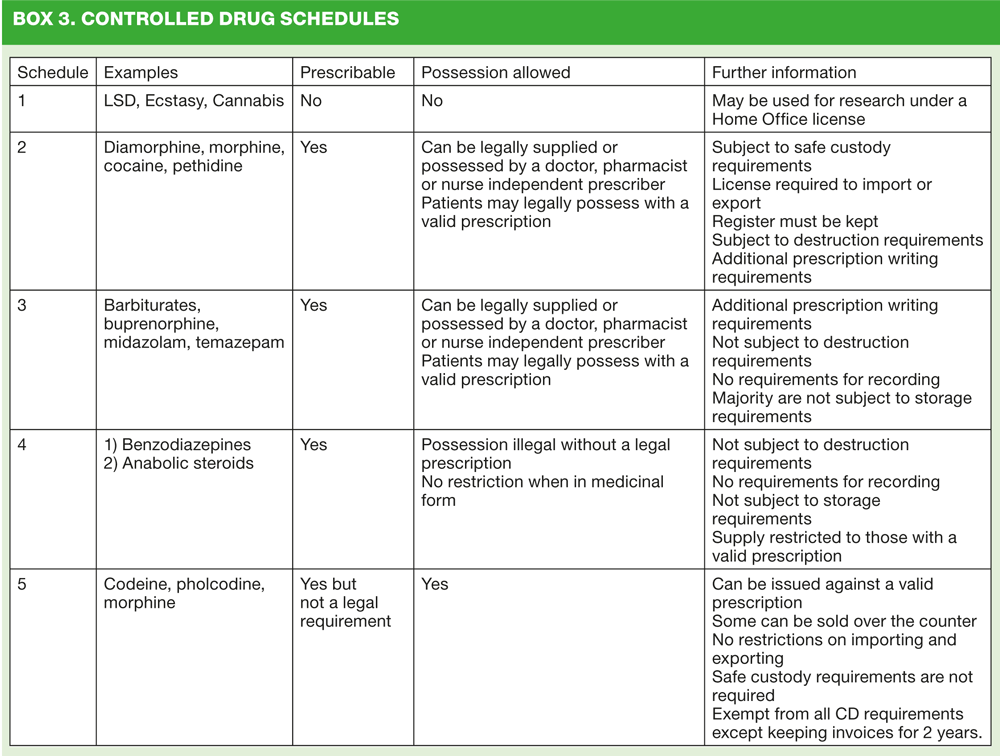
The Misuse of Drugs Act (1971) was amended in 2001 to detail when it is lawful to have possession of or to supply controlled drugs when acting in a professional capacity. This regulation divides the drugs into five schedules relating to their therapeutic usefulness or misuse potential, with schedule 1 listing those drugs with little or no therapeutic value (often drugs used illegally for recreational use) and schedule 5 listing those that in some cases may be bought over the counter. The schedules also detail the requirements governing the import and export, production, supply, possession, prescribing and record keeping.2 (Box 3).
The Shipman effect
Following the Shipman Inquiry Fourth Report3 in 2004 and subsequent recommendations, a substantial programme of work was set out by the Government in 'Safer Management of Controlled Drugs - the Government's Response to the Fourth Report of the Shipman Inquiry (2004)'.4 It looked at improving and strengthening controlled drugs management, while recognising that patients still need to be able to access the medications they require in a timely manner without compromising health professionals' legal responsibilities. One of the key elements to this is the requirement of health bodies to appoint an Accountable Officer whose main responsibility is to secure the safe management and use of controlled drugs.
Further amendments were made in 2006 and 2007, to address the requisition requirements of controlled drugs in the community.5,6 A Department of Health circular (027/2007) specified the tighter regulation of CDs with complete audit traceability; the amendment also enabled the Accountable Officer - as the person responsible for the safe use and management of controlled drugs in his or her healthcare area - to nominate people to witness the destruction of controlled drugs. This requirement is particularly relevant to healthcare professionals who may visit palliative care patients in their homes and be asked to dispose of, or remove medications. It is essential that if this is part of your role you are fully aware of your local requirements and procedures and that you discuss any queries with your local Accountable Officer.
Restrictions lifted
The key change resulting from the 2012 statutory instrument is the lifting of all restrictions to independent non medical prescribing of controlled drugs in schedules 2-5, the exception to this is diamorphine, cocaine and dipipanone for the treatment of addiction. However, nurses working in substance misuse can now prescribe other controlled drugs for the treatment of addiction.1
The regulations allow independent prescribers to mix two or more controlled drugs to be administered to a patient, or to provide written authority to another to do so. The regulations also allow supplementary prescribers to mix drugs under the terms of a clinical management plan. It is essential that all prescribers are aware of the potential for drug interactions and have the clinical knowledge to combine controlled drugs safely within their competence.1
A PGD is a written direction relating to the supply and or administration of a prescription or class of prescription only medicine. In the majority of cases, care should be provided on an individual, patient-specific basis, and using PGDs should be reserved for situations where they would benefit patient care (within appropriate professional relationships and accountability).7 Until the new regulations came into force, diamorphine and morphine could only be supplied or administered on a PGD for cardiac pain in a hospital emergency department or coronary care unit: however, this restriction has now been lifted and allows these drugs to be given for the immediate necessary treatment of sick or injured patients (excluding addiction).
SAFE AND EFFECTIVE PRESCRIBING
'The prescription of medicines is the most common form of therapeutic intervention in medicine and therefore the quality of that prescription is a foundation stone for high quality patient care'.8 As such the process of prescribing controlled drugs should be exactly the same as for any other drug, regardless of its legal category.
Safe and effective prescribing is often a complex process that impacts not only on the patient but also the prescriber and the NHS, often with many factors having to be considered before the prescription is written.9 For every non medical prescriber, a fundamental element of the education programme is learning principles for good prescribing practice. Many will be familiar with the National Prescribing Centre 'Signposts for prescribing nurses - general principles of good prescribing',9 which describes seven principles to aid prescribing decision making.
Before prescribing for any patient a holistic assessment of the patient should be undertaken, which should include medical, social and drug history with particular attention being paid to OTC or herbal preparations being taken, together with any known drug allergies. Pertinent to CD drug prescribing is that codeine products in the form of co-codamol or codeine linctus are readily available over the counter, as is kaolin and morphine mixture, and these could put the patient at significant risk of adverse effects if combined with other opiate medications. Many pharmacists use a mnemonic when recommending over the counter preparations such as WWHAM, ASMETHOD and ENCORE, (Box 4) these all encompass checking for other medications being taken and this may be a strategy to employ to ensure this is not overlooked.
Using a holistic approach allows you to consider the most appropriate product for that individual patient; choices for children will differ from those for the elderly patient, which may differ again from those for a dying patient.
When considering the appropriate strategy and negotiating a contract to achieve concordance, a prescription is often only one of the options available and non pharmaceutical options may be more appropriate and agreeable to the patient.
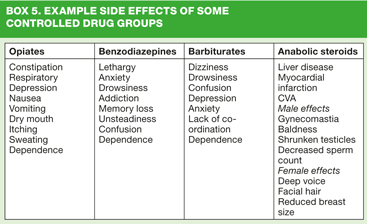
With all medications, the lowest effective dose should be given to avoid adverse reactions and a full discussion of any potential side effects is essential. Each patient should be reviewed regularly and included in this should be a review of side effects patient may be experiencing or any difficulties in taking the medication as prescribed. (Side effects of some classes of CDs are given in Box 5)
As a responsible professional any medication prescribed or recommended should be documented clearly in the patient's record as soon as possible. (Practice Nurse, 25 May 2012).
In May 2012 the National Prescribing Centre (NPC) published a new single competency framework for all prescribers.8 The aim of this framework is to ensure healthcare professionals are safe and effective prescribers. The framework is designed to be used from a new prescriber in training through to experienced prescribers and covers both medical and non medical prescribers. It is split into three domains, the consultation, prescribing effectively and prescribing in context, each with three competency dimensions within them.
- The consultation: knowledge; options; shared decision making
- Prescribing effectively: always improving; professional; safe
- Prescribing in context: the healthcare system; information; self and others.
The NPC framework provides an ideal vehicle for continuing professional development and reflecting on prescribing practice. Using this framework can identify areas of strengths and weaknesses to enable the conditions of the NMC Code to be met,11 while maintaining safe and effective prescribing practice to your patients.
PRESCRIBING REQUIREMENTS FOR CONTROLLED DRUGS
The requirements relate to all schedule 2 and 3 drugs except temazepam.
The prescription must be either written in indelible ink or computer generated. They must be signed by the prescriber, dated and specify the prescriber's address. (Figure 1)
The prescription must clearly state:
- The patient's full name, address and age where appropriate
- The name and form of the drug (e.g. tablets, even if only one form exists)
- The strength of a preparation (where appropriate)
- The dose to be taken
- The total quantity to be supplied in words and figures, noting that it is strongly recommended that supply is for no longer than 30 days
- Prescriptions for schedule 2, 3, and 4 drugs are only valid for 28 days
Requests for emergency supplies of schedule 2 and 3 CDs for a specific patient, requested either by the patient or the practitioner are not allowed, with the exception being phenobarbital for epilepsy.5
DRUG ADDICTS
Addicts can employ a variety of strategies to try to obtain controlled drugs to feed their habit, and as a prescriber it is essential you are aware of, and alert to, this potential deception.
Be aware of the commonest drugs being abused: the most frequently misused drugs are cocaine, diamorphine, morphine and the synthetic opiates. Benzodiazepines are also widely misused; however barbiturate abuse is much less common.
The BNF (2012)2 suggests addicts may attempt to visit several prescribers (using different names, re-registering at different practices), attempt to forge prescriptions and fabricate stories such as lost prescriptions or medication.
To help prevent inadvertent supply to addicts, ensure you:
- Draw a diagonal line at the bottom of the prescription to prevent additions
- Look back at previous issues and dosages
- Be aware of gradual but persistent increases in dosing
- Avoid alterations on prescriptions
- Follow the controlled drug prescribing guidelines
- Clearly document in the patient's record
CONCLUSION
Welcome changes to the controlled drug regulations allow professionals to provide holistic care to patients in a timely manner. For this process to be safe and effective, prescribers should have a working knowledge of the legal requirements related to controlled drugs and their professional body's requirements, ensure they work within their scope of clinical practice and individual boundaries, be aware of the potential for controlled drug abuse, and put robust processes in place for continuing professional development and reflection.
REFERENCES
1. The Home Office (2012) The Misuse of Drugs (Amendment No.2) (England, Wales and Scotland) Regulations. Statutory instrument No. 973. The Home Office; London
2. British Medical Association and the Royal Pharmaceutical Society (2012) British National Formulary 63. BMA & RPS; London
3. Department of Health (2004) Shipman Inquiry Fourth Report - The Regulation of Controlled Drugs in the Community. DH; London
4. Department of Health (2004) Safer Management of Controlled Drugs - The Governments response to the Fourth Report from the Shipman Inquiry http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4097904 accessed 22/06/12
5. The Home Office (2006) The Controlled Drugs (Supervision of Management and Use) Regulations Statutory Instrument No.3148 http://www.legislation.gov.uk/uksi/2006/3148/pdfs/uksi_20063148_en.pdf accessed 20/06/12
6. The Home Office (2007) Misuse of Drugs and Misuse of Drugs (Safe Custody) (Amendment) Regulations Circular No. 027/2007 http://www.homeoffice.gov.uk/about-us/corporate-publications-strategy/home-office-circulars/circulars-2007/027-2007 accessed 20/06/12
7. Royal College of Nursing (2006) Patient Group Directions - guidance and advice for Nurses. http://www.rcn.org.uk/__data/assets/pdf_file/0008/78506/001370.pdf accessed 24/06/12
8. National Prescribing Centre (2012) Single Competency Framework for all Prescribers. National Prescribing Centre; Liverpool
9. National prescribing Centre (1999) Signposts for prescribing nurses - general
principles of good prescribing. Prescribing Nurse Bulletin. 1;1
10. National Institute for Clinical Excellence (2009) Low back pain - early management of persistent non-specific low back pain. www.nice.org.uk/CG88 assessed 18/6/12
11. Nursing and Midwifery Council (2008) The Code: Standards of conduct, performance and ethics. NMC; London
12. Nursing and Midwifery Council (2008) Record keeping: Guidance for nurses and midwives. NMC; London