New flu vaccine and potential delays to start of flu campaign
Mandy Galloway,
Mandy Galloway,
Editor
A new, and it is claimed, more effective flu vaccine can be used in the forthcoming flu season but delays to the announcement about which strains should be included in this year’s vaccine may mean practices have to start their campaign late
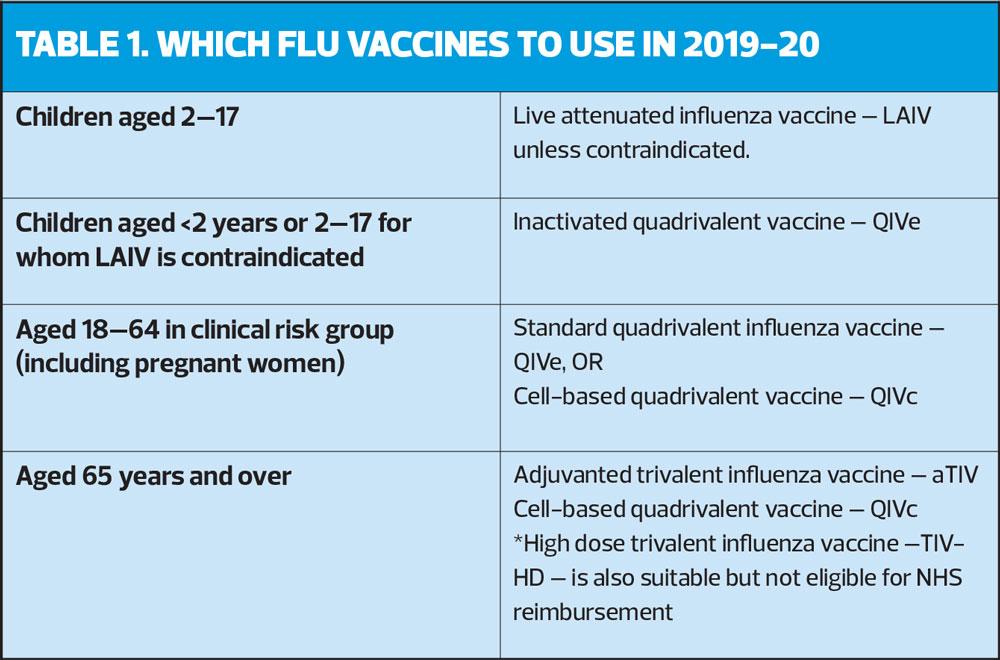
This flu season is the first that an innovative cell-based flu vaccine, effective against four strains of flu, comes into general use for people aged 18 to 64 years in a clinical risk group, and the over-65s.
While the standard egg-grown quadrivalent influenza vaccine (QIVe) is still available for use in the clinical risk groups, the newly licensed cell-based quadrivalent influenza vaccine, QIVc is estimated to be over 36% more effective than standard vaccines.
Research has shown that some H3N2 viruses undergo changes when they are grown in eggs – a process known as egg-adaptation – and this is thought to reduce the effectiveness of standard egg-based influenza vaccines in H3N2-dominated seasons. When the H3N2 component of cell-based vaccines are produced completely outside of the egg-based process, it may offer a closer match and potentially improved protection against the circulating H3N2 strain compared with standard egg-based vaccine.
Flucelvax Tetra® is the first cell-based influenza vaccine, offering protection against four strains of the flu virus.
There have been no randomised controlled trials comparing the efficacy of QIVc and QIVe but an analysis of data from the medical records of more than 1.3m patients in the US concluded that QIVc was more effective than QIVe in preventing influenza-like illness (relative vaccine efficacy, 36.2%, p<0.001).
Last year (2018-19) saw the introduction of the adjuvanted tricyclic influenza vaccine (aTIV), Fluad® which has been shown to offer more effective protection against flu in older adults, who do not produce as good an immune response to vaccination as younger people. In previous seasons, the conventional inactivated flu vaccine showed ‘no significant efficacy in the elderly against the A (H3N2) influenza virus. This subtype of the virus is associated with outbreaks even in highly-vaccinated populations, such as the residents of care homes, and typically results in excess mortality. aTIV is still available for 2019-20, and is recommended for patients over 65 years.
Table 1 shows which vaccines should be administered this season to each eligible group.
Effectiveness of last year’s flu vaccine against all strains was estimated to be:
- 44.3% (95% CI 26.8, 57.7) across all ages
- 48.6% (95% CI -4.4, 74.7) for 2-17 year olds (LAIV only)
- 44.2% (95% CI 21.3, 60.5) for 18-64 year olds
- 49.9% (95% CI -13.7, 77.9) for those aged 65 and over (all vaccine)
- 62% (95% CI 3.4, 85.0) for those aged 65 and over (aTIV only)
VACCINE COMPOSITION 2019-20
Each year, the composition of flu vaccine for the northern hemisphere is recommended by the WHO, based on the strains circulating in the southern hemisphere in the previous winter season. The final recommendations were released a month late, in March 2019:
- An A/Brisbane/02.2018 (H1N1)pdm09-like virus
- An A/Kansas/14/2017 (H3N2)-like virus
- A B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage); and
- A B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage)
Potential delays
Industry bodies have warned that – as it takes 6-8 months to produce and approve vaccines once the candidate strains have been announced – the delayed announcement may cause a knock-on delay in delivery of supplies, and a delay of up to two months to the start of the 2019-20 campaign.
Sanofi is among the manufacturers warning that flu vaccine supply was expected to be ‘significantly weighted towards the fourth quarter’.
However, QIVc is less likely to be affected: unlike egg-based production, which requires roughly one egg for each vaccine dose, cells used for cell-based culture can be kept frozen in storage, allowing for production to be rapidly scaled up.
UPTAKE LAST SEASON
Uptake of flu vaccine for 2018-19 in general practice was:
- 72% for the over 65s, down from 72.9% in 2017-18
- 48% in at risk groups, down from 49.7%
- 45.2% in pregnant women, down from 47%
- 44.9% in 2- and 3-year-olds, up from 44%
There was wide variation in uptake in the over-65s between CCG areas, ranging from 56.5% (Hammersmith and Fulham CCG) to 81% (Rushcliffe CCG). Just 30 out of the 195 CCGs achieved the WHO target of 75% (less than last season, where 48/195 CCGs met the 75% target). Although these percentages appear to show a decline the number of vaccines given increased to more than 7.5 million.
In the at risk groups, the lowest uptake was in patients with morbid obesity (BMI ≥40), at 34.7%. Again, although the percentage vaccine uptake has decreased for all individual clinical risk groups, the number of patients in each group increased by more than 2%, except those with chronic respiratory disease, which remained stable.
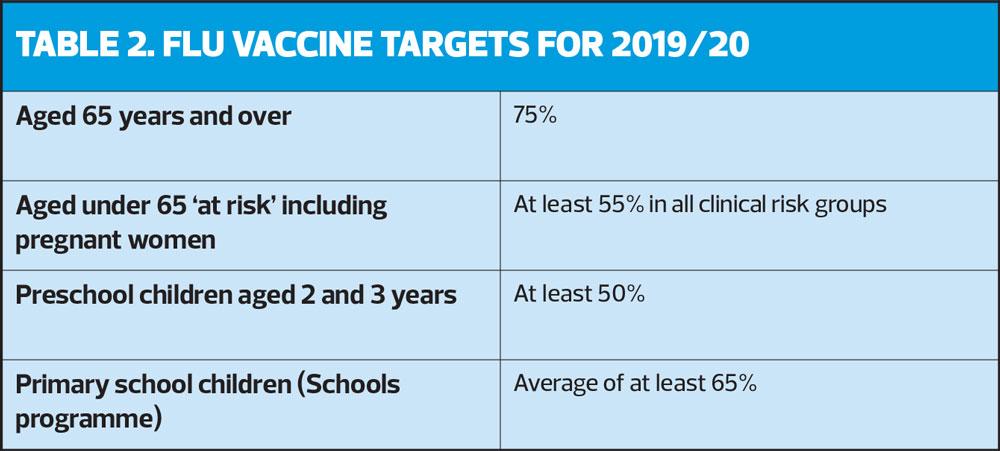
Targets for this season are shown in Table 2.
Related articles
View all Articles