Opioid-induced bowel dysfunction
Mandy Galloway and Rachel Booker
Mandy Galloway and Rachel Booker
Patients with moderate to severe pain are frequently treated with opioid analgesics. However, the efficacy of these drugs can be limited by common - and sometimes severe - gastrointestinal side effects
The treatment of chronic pain frequently involves the use of opioids, with some epidemiological studies estimating that opioids are used in up to 90% of patients with chronic, moderate to severe pain.1 However, their analgesic efficacy can be limited by side effects.
Moderate to severe pain is estimated to affect almost one in five adults, with many people experiencing the problem for anywhere from two to 15 years.2 The majority of these patients (70%) are managed in general practice, and more than half are taking prescribed medication to alleviate their pain.2 Severe back pain alone accounts for 50 million days off work, and £500 million in NHS treatment costs each year.3
The most commonly used prescription-only analgesics are non-steroidal anti-inflammatory drugs (NSAIDs), but according to a survey of more than 46,000 chronic pain sufferers in Europe, 5% are treated with strong opioids.4
As such, most practice nurses will regularly see at least some patients who are prescribed opioid drugs to control moderate to severe pain. And, following amendments to the Misuse of Drugs Act 1971 in April 2012, suitably qualified nurses can now prescribe opioids and other controlled drugs (CDs).
As with all medications, the lowest effective dose should be given to avoid adverse reactions and a full discussion of any potential side effects is essential. Each patient should be reviewed regularly and included in this should be a review of side effects patients may be experiencing or any difficulties in taking the medication as prescribed.
MODE OF ACTION
Opioids work by binding to opioid receptors. These are found principally in the central and peripheral nervous system, where they mimic the action of naturally occurring endorphins, leading to decreased perception of pain, decreased reaction to pain, and increased tolerance of pain. They provide effective relief of both malignant and non-malignant pain, such as post-operative, musculoskeletal (including inflammatory and degenerative arthritis), and to a lesser extent, neuropathic pain.
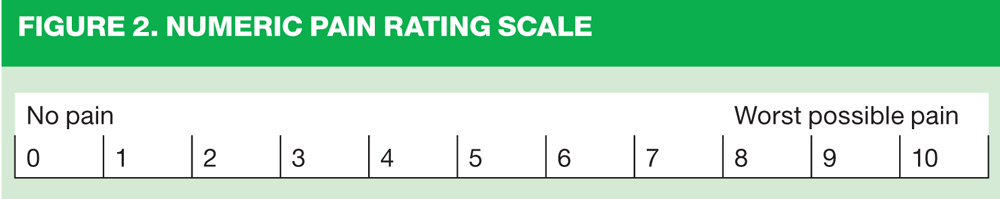
In line with the World Health Organization analgesic ladder, (Figure 1)5 opioids are deployed when simple analgesics ± NSAID ± adjuvants (e.g. tricyclic antidepressant in neuropathic pain) are ineffective. For mild to moderate pain (3 – 6 on a numerical rating scale, see Figure 2) the next step is to introduce a weak opioid, such as codeine, in combination with a non-opioid analgesic. For moderate to severe pain (>6 on a 11-point numerical rating scale, Figure 2) oral morphine is recommended, but alternative opioids, including diamorphine, buprenorphine, fentanyl, methadone and oxycodone may also be used in some circumstances.
ADVERSE EFFECTS
While opioid analgesics are the gold standard therapy for most patients in moderate to severe pain, their efficacy may be limited by adverse effects. (See Box 1)
In addition to the adverse effects of sedation and respiratory depression, the most common side effects of opioids are constipation, nausea and vomiting. Whereas nausea and vomiting will wear off in most people within a week to ten days, tolerance to constipation seldom develops. This can be severe and debilitating and, for many patients, is sufficiently troublesome to cause them to stop treatment as the discomfort of the constipation outweighs their pain. In one survey, up to a third of patients reported missing, decreasing or stopping use of opioids in order to make it easier to have a bowel movement.6
The reason that the gastrointestinal (GI) tract is such a significant site of opioid-related adverse effects is because it has an extensive network of enteric neurons. These, as well as supplying nervous stimulation to all parts of the alimentary canal, are involved in the control of many other body functions, including immune, sexual and behavioural functions.1 Three major classes of opioid receptors are located in the enteric nervous system: when opioid agonists bind to these receptors, they regulate bowel function, influencing both secretion and motility. Typically, endogenous opioids will slow down an overactive gut, but having restored normal function they are quickly metabolised.1
OPIOID-INDUCED BOWEL DYSFUNCTION
In contrast, exogenous opioids continue to disrupt bowel function, by interrupting peristalsis (the co-ordinated muscular contractions responsible for GI transit), reducing secretions into the gut and increasing reabsorption of fluid from the gut (as the stool remains in the intestine for longer). These effects lead to signs and symptoms such as:
- Constipation
- Decreased gastric emptying (leading to gastro-oesophageal reflux and heartburn)
- Abdominal cramping
- Spasm
- Bloating
- Delayed GI transit
- Formation of hard, dry stools
- Nausea and vomiting2
Together, these signs and symptoms are now recognised as opioid-induced bowel dysfunction (OBD) and, despite the fact that OBD symptoms may persist for as long as opioid administration continues, its impact on patients is often underestimated.7
As we have seen, the most common and debilitating symptom of OBD is constipation, which affects between 15% and 90% of patients receiving opioids for non-cancer pain,2 and between 70% and 100% of patients receiving cancer treatment.7 Constipation is more prevalent in patients treated with strong opioids, e.g. transdermal fentanyl and sustained release morphine, than among those prescribed weak opioids such as codeine.2
The wide variation in reported incidence may be explained, in part, by the variety of definitions of constipation used by both patients and healthcare professionals: some patients consider that they are constipated if they do not have a bowel movement every day, whereas others expect only three movements per week. However, an acceptable definition can be remembered using the acronym DISH:7
- Difficult to pass
- Infrequent compared with normal
- Smaller than normal, and
- Hard
The diagnostic criteria for constipation are set out in Rome III,8 and not only encapsulate bowel movement frequency, but also the discomfort associated with constipation (Box 2). However, the Rome III definition was developed to describe functional constipation (FC), and the management of OBD requires different approaches.6
MANAGEMENT
There are no widely accepted guidelines for the management of OBD,2 but the European Association of Palliative Care Research Network recommends the following strategies for the management of adverse effects associated with oral morphine:
- Reduce the dose
- Rotate (switch) opioids
- Manage symptoms
Until recently, treatment of opioid-induced constipation has been based on the treatment of functional constipation, i.e. with laxatives or bulk forming agents, but it is estimated that only 46% of patients with OBD treated with laxatives achieve relief of symptoms more than half of the time.9
Co-prescription of laxatives has been reported to improve bowel movements in many patients but not for everyone, and so attention has been given to the role of opioid receptor antagonists such as naloxone. A potential drawback of adding an opioid antagonist is that it may reduce analgesia in some patients, but in a study of more than 7,800 patients, pain was reduced on average by 2.9 points on an 11-point numeric rating scale, and bowel function improved significantly, with a decrease in constipation from 71% at the start of the study to 34% at four weeks.10
A more recent study has confirmed that a prolonged release combination of oxycodone and naloxone offered effective analgesia while improving OBD in patients with chronic pain.11
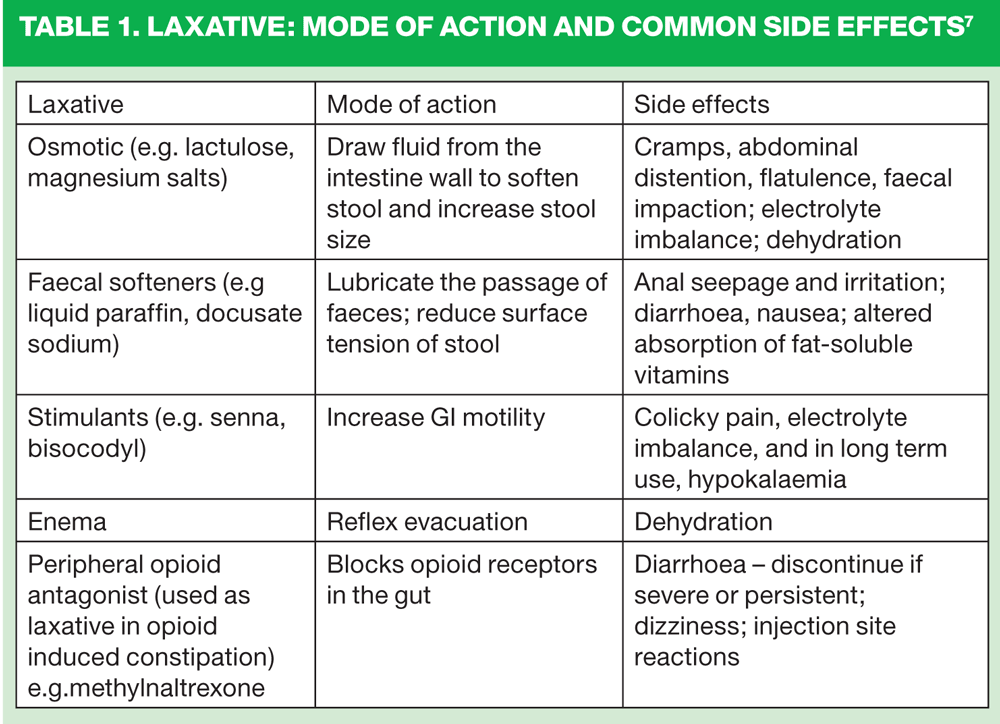
This approach has the potential to decrease the use of laxatives,9 which are not themselves without adverse effects (Table 1).2,7
A stepwise approach to management of OBD
- Avoid bulking agents, which in OBD, may increase bloating and flatulence, and increase absorption of water from the gut, leading to further hardening of the stool
- Start treatment with a bowel stimulant and/or stool softening agent
- If unsuccessful, consider switching to a combined opioid/opioid antagonist
CONCLUSION
One of the greatest drawbacks of opioid analgesics is that they disrupt bowel function, due to the presence of opioid receptors in the gut. This can frequently lead to decreased secretions into the large intestine, and increased reabsorption of fluid from the stool, slower GI transit and decreased gastric emptying. The most common symptom is constipation, accompanied by abdominal cramping, and the formation of hard dry stool. This group of signs and symptoms are now recognised as opioid-induced bowel dysfunction, which may persist as long as opioids are administered. Constipation may be alleviated by the use of laxatives, but neither osmotic nor bulk-forming agents are recommended as they may exacerbate bloating and discomfort: if a laxative is used, either a faecal softener or stimulant agent is preferred. However, use of a laxative does not tackle the underlying problem of activation of opioid receptors in the GI tract, and the introduction of an opioid-antagonist should be considered. The combination of oxycodone+naloxone has been shown to provide satisfactory levels of analgesia with a meaningful improvement in bowel function over a 4-week treatment period.10
TEST YOUR KNOWLEDGE
Visit practicenurse.co.uk/Curriculum for a 10-question module on the management of moderate-to-severe pain: complete the resource to earn a certificate for 1 hour of continuing professional development to include in your annual portfolio.
REFERENCES
1. Peppin JF. Opioid-induced constipation: causes and treatments. Practical Pain Management, 2012. Available at: http://www.practicalpainmanagement.com/opioid-induced-constipation-causes-treatments
2. Panchal SJ, Muller-Scwefe P, Wirzelmann JI. Opioid induced bowel dysfunction: prevalence, pathophysiology and burden. Int J Clin Pract 2007;61:1181-87
3. Little P et al. General practitioners’ management of acute back pain: a survey of reported practice compared with clinical guidelines. BMJ 1996;312:485-488
4. Breivik H, Collet B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287-333
5. World Health Organization. Cancer Pain Relief, 2nd edn. Geneva: WHO, 1996
6. Bell TJ, Panchal SJ, Miaskowski C. The prevalence, severity and impact of opoid-induced bowel dysfunction: results of a US and European patient survey (PROBE 1). Pain Medicine 2009;10:35-42
7. McMillan SC. Assessing and managing opiate-induced constipation in adults with cancer. Cancer Control 2004;11:3-9
8. Rome III Diagnostic criteria for functional gastrointestinal disorders. http://www.romecriteria.org/assets/pdf/19_RomeIII_apA_885-898.pdf
9. Pappagallo M. Incidence, prevalence and management of opioid bowel dysfunction. Am J Surg 2001;182 (Suppl):11S-18S
10. Schutter U, Grunert S, Meyer C, et al. Innovative pain therapy with a fixed combination of prolonged-release oxycodone/maloxone: a large observational study under conditions of daily practice. Curr Med Res Opin 2010;26:1377-87
11. Mercadente S, Giarratono A. Combined oral prolonged-release oxycodone and naloxone in chronic pain management. Expert Opin Investig Drugs 2013;22:161-6
Related articles
View all Articles