The importance of achieving target HbA1c in the early years
JUDY DOWNEY
JUDY DOWNEY
RN, BSc (Hons)
Diabetes Educator Practitioner, Hammersmith and Fulham GP Federation
Achieving good glycaemic control in the early years after diagnosis of type 2 diabetes reduces the time spent in a hyperglycaemic state and thus decreases the ‘glycaemic legacy’ effect and so can be expected to lessen or delay the occurrence of complications
The incidence of type 2 diabetes in the UK is rising rapidly. There are 3.5 million people in the UK diagnosed with diabetes (90% of whom have type 2 diabetes) and it is estimated that a further 1.1 million people have undiagnosed diabetes.1 This equates to 25% of the UK population who live with, or who are at risk of developing diabetes. By 2025 it has been predicted that 5 million people in the UK will have diabetes.1 This unprecedented increase in numbers of people with diabetes, the majority of whom are now solely managed by primary care, will inevitably lead to huge increase in costs to the NHS.
In 2015 it was estimated that the NHS spent about £10 billion – 10% of the budget on diabetes. The total cost associated with diabetes in the UK stood then at £23.7 billion and is predicted to rise to £39.8 billion by 2035–6.1 A significant proportion of this cost is spent on managing complications that are largely preventable with good glycaemic control.2 The United Kingdom Prospective Study Group (UKPDS) data informed us that improving glycaemic control in diabetes, will reduce and/or delay the occurrence of micro and macro-vascular complications.3 More recently we have learnt that achieving tight glycaemic control in the early years following diagnosis, will significantly reduce risk of complications later in the course of the disease. Time spent in a hyperglycaemic state is known as the glycaemic legacy.4
CLINICAL INERTIA
So why is it, that this early intensification of therapy to reach agreed HbA1c targets does not occur? Clinical inertia is the term used to describe the failure of the clinician to intensify treatment in a timely manner.5 When pharmacological treatments, be they oral hypoglycaemic or injectable therapies, are not initiated or are delayed, a person’s quality of life and their long-term health outcomes are adversely affected. Clinical inertia is a significant factor for inadequate glycaemic control and may be influenced by the clinician’s own judgement, experience and knowledge of guidelines.6
GLYCAEMIC TARGETS
Control of blood glucose levels is a key area in the management of people with type 2 diabetes. Healthy lifestyle change – including weight control, increase in exercise and smoking cessation – underpins every aspect of diabetes management. In diabetes care, it is vital to take every opportunity to discuss lifestyle issues with patients, advising them that the oral and injectable therapies they are prescribed are much less effective if lifestyle changes are not made and maintained.
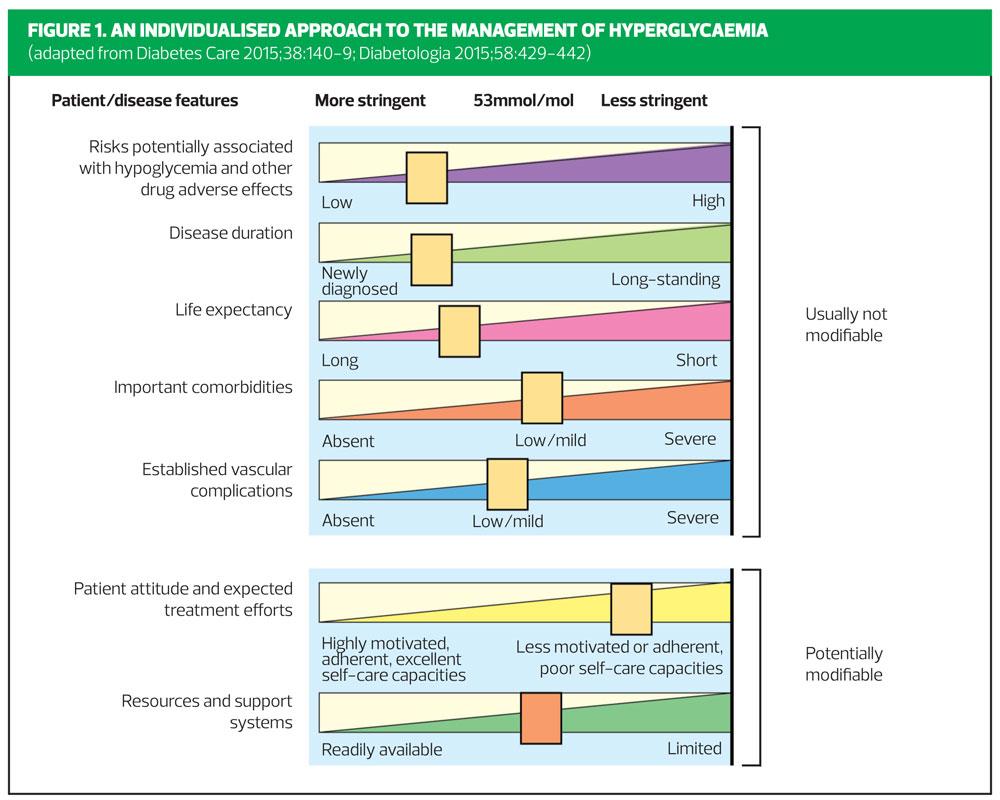
It can be quite challenging to discuss glycaemic targets with patients, but Figure 1 provides a visual guide to the seven modifiable and non-modifiable factors that need to be taken into account when agreeing HbA1c targets.
Reducing HbA1c decreases the onset and progression of microvascular complications such as retinopathy.7 The impact on macrovascular complications remains less certain, possibly only showing itself after many years of improved glycaemic control.8
In agreeing glycaemic targets with patients, it is vital to consider the risks and benefits, balancing the benefits of better glycaemic control against potential risks such as hypoglycaemia. We should bear in mind that the lower the HbA1c, the greater the risk of hypoglycaemia. Individualisation is the key, taking into account potential side-effects of medication, such as hypoglycaemia, and the patient’s age and state of health, alongside other factors such as comorbidities, life expectancy, mental health issues and support systems in place.
Diabetes UK figures show the incidence of type 2 diabetes in 2012–2013 was 11% in those aged 40 – 49, and as high as 19% in those aged 50–59. For those in their 60s, the incidence has risen to 26%.1 These numbers will certainly be higher in 2017-18.
When setting HbA1c targets for people aged 40–49, who are newly diagnosed, without co-morbidities and have many years life-expectancy, one might aim for a much more stringent HbA1c target, than for a frail, older person in which the risks of tight glycaemic control might exceed the benefits.
CONSIDERATIONS IN NEWLY DIAGNOSED PATIENTS
NICE guidelines for management of type 2 diabetes, published in December 2015 and revised in 2017, advise that we should support adults to aim for an HbA1c target of 48 mmol/mol, rising to 58mmol/mol as treatment intensifies.9 For the younger patient population, who have been diagnosed for less than 5 years, and who have no comorbidities, aiming for an HbA1c of 48 mmol/mol will, if achieved, reduce their chances of developing complications many years later – the legacy effect.
Lifestyle changes remain an important topic at all times for patients with type 2 diabetes – newly diagnosed patients should be supported to make healthier lifestyle choices, as these are as important, if not more so, than any therapy that is prescribed.
NICE recommends that all newly diagnosed patients should be offered structured education at the time of diagnosis – group education is the preferred format – but with uptake at around 70%, there is room for improvement.10
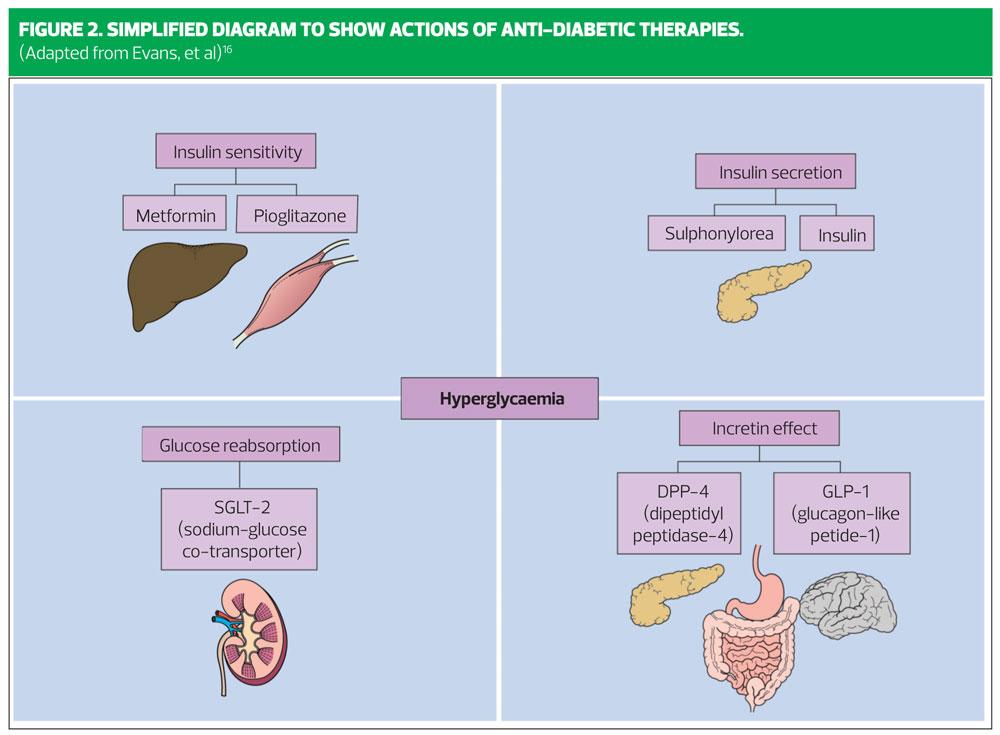
At diagnosis of type 2 diabetes, where the HbA1c rises above 48 mmol/mol, up to 50% of beta cell function has already been lost.8 Consideration should be given to this fact when selecting an add-on therapy to metformin. Figure 2 shows a simplified guide to the pharmacological actions of different classes of diabetes therapy.
NICE recommends using metformin as the first line therapy, starting at a low dose and titrated to 1 gram BD.9
Metformin belongs to the class of drug called biguanide – its mode of action is to suppress liver glucose output, and also to enhance insulin sensitivity in the liver. It does not stimulate insulin secretion.
It is important to increase the dose of metformin slowly to minimise the chance of gastrointestinal side-effects. A sustained release version is available for those patients who have problems with gastrointestinal side-effects.
WHAT NEXT AFTER METFORMIN?
The addition of further agents to metformin, with the aim of reducing the HbA1c to 53 mmol/mol, as recommended by NICE, is often known as the stepwise approach to therapy. Stepwise management is a reactive strategy, but waiting until HbA1c levels rise to the next ‘trigger point’ before intensifying therapy can result in extended periods of hyperglycaemia, increasing the glycaemic legacy – and we know that even short periods of hyperglycaemia increase the risk of microvascular and macrovascular complications.12
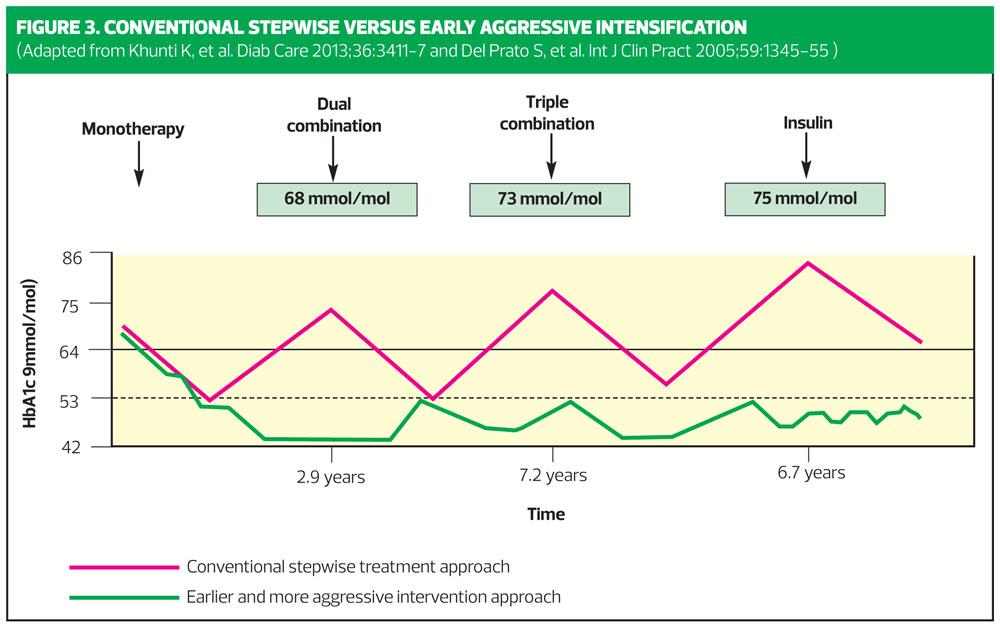
Figure 3 shows conventional stepwise treatment approach, that many of us are used to – intensification of therapy (in red) delayed until HbA1c rises to around 9% or 76 mmol/mol VS earlier and more proactive approach (in green), where each intensification is made when HbA1c rises to between 53 and 58mmol/mol.
Earlier introduction of combination therapy offers the potential for therapeutic goals to be achieved more rapidly than with conventional stepwise management, thus reducing the risks associated with extended periods of poor glycaemic control.
In my experience, combination therapy with smaller doses of oral hypoglycaemic agents may offer improved safety and tolerability, compared with monotherapy with a single agent titrated to maximal doses.
Combination therapy with agents with complementary mechanisms of action is likely to have additional benefits for the long-term management of type 2 diabetes.
Both current and previous versions of NICE guidance on type 2 diabetes have established metformin as first-line therapy, with a sulfonylurea as the first add-on agent. But in the last decade, several other classes of therapy have been introduced, including the injectable GLP-1 receptor agonists, DPP4 inhibitors and SGLT2 inhibitors. NICE guidelines now give the choice of all four therapies when intensifying therapy after metformin.
- SGLT2 inhibitors
- DPP4 inhibitors
- Pioglitazone
- Sulfonylureas (SUs), and
- Insulin, where indicated
For example, a second-line addition to metformin now could be a DPP4 inhibitor or pioglitazone (which targets insulin resistance), or an SGLT2 inhibitor (promotes excretion of glucose in urine, thereby lowering blood sugars).
Gliclazide is the SU most widely used in the UK, and remains a useful first or second-line therapy if a person is very symptomatic of hyperglycaemia. It can be used as a temporary measure, because it rapidly lowers blood glucose – a characteristic which means it is also associated with an increased risk of hypoglycaemia. Once hyperglycaemic symptoms have resolved, the SU can be stopped as another agent is introduced to minimise the risks of hypoglycaemia and of weight gain which occurs with extended use.
Let us consider now, how to select a therapy to be used second line after metformin. As already mentioned, NICE guidance now lists a choice of four drug classes to be considered at this stage:
- Metformin + DPP4, or
- Metformin + pioglitazone, or
- Metformin + sulphonyurea, or
- Metformin + SGLT2.
NICE states that no priority is intended by the order in which the four classes are listed. Thus, DPP4 inhibitors, SGLT2 inhibitors, pioglitazone and SUs are recommended as co-equal classes, from which clinicians can choose to add to metformin.
This degree of choice for the non-specialist can be daunting, but you can find excellent summaries to help you select the most appropriate class of drug – and to choose within class – in Diabetes Masterclass: Optimising therapies for type 2 diabetes, Practice Nurse August 2015.
Initially we should aim to reach an HbA1c target of 48 mmol/mol with monotherapy, but NICE guidance suggests the first intensification is made when the HbA1c rises to 58 mmol/mol. When considering each case on individual merits, it is important to consider if it is appropriate to wait until the HbA1c rises to 58 mmol/mol in our younger patients.
Assessment each of the seven factors shown in Figure 1 will assist with this decision. In an older patient with comorbities and established vascular complications, with poor self-care capacities, or with lower life expectancy it may be more appropriate to aim for a less stringent target, of 53mmol/mol.
The important ACCORD study,13 where the majority of participants were of high cardiovascular risk with long-standing diabetes, has changed our practice so that now, driving down HbA1c to 48 mmol/mol in such patients is considered inappropriate – but then, persisting with very poor glycaemic control is also unsafe. ACCORD did not demonstrate that all patients with long-standing disease should have poorer glycaemic control, but suggested less stringent treatment goals for those with established cardiovascular disease and other important comorbidities.
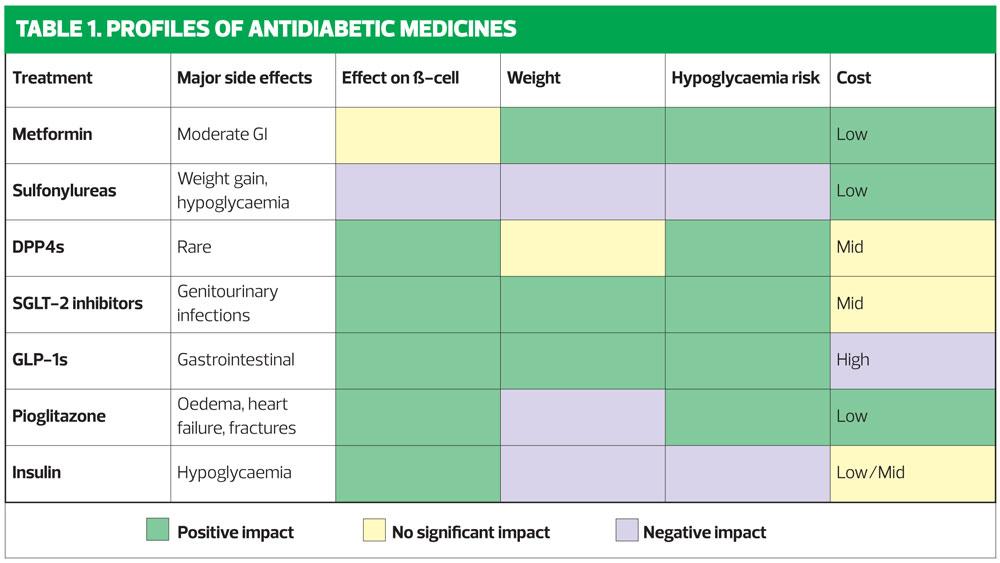
When choosing add-on therapies, the avoidance of hypoglycaemia is an important consideration: some of the newer therapies, used in addition to metformin, are less likely to cause hypoglycaemia than SUs or insulin.
Weight gain is an undesirable consequence of both SUs and insulin, and to some extent Pioglitazone: the GLP-1s and SGLT2s are not associated with weight gain, and indeed are likely to result in weight loss.
NICE only recommends GLP-1s for patients in whom triple therapy is not effective and who have a BMI of 35kg/m2 or for whom insulin therapy would have significant implications for their occupation – or where weight loss would benefit other significant obesity-related comorbidities. This ‘ranking’ is due, at least in part, to their cost. In practice, weight loss is essential for many overweight patients to achieve their HbA1c target. Table 1 summarises the characteristics of the available drug classes, in terms of their effect on weight, the risk of hypoglycaemia and their cost.
IN SUMMARY
In management of type 2 diabetes, clinicians and patients should aim to reach agreed HbA1c target, depending on individual patient characteristics, and aim to keep glycaemic control at or near to target in the first 2–5 years following diagnosis. Patient education and lifestyle measures are a vital component of diabetes management. When intensifying therapy, long-term outcomes will be improved if HbA1c is not allowed to rise above pre-set target, which in younger patients should be 48 mmol/mol. If HbA1c does rise above target, a second line agent should be introduced sooner rather than later, and consideration should also be given to introducing a third agent if the target HbA1c is proving hard to achieve. Therapies are available, that will help many patients to achieve glycaemic control without increasing their risk of hypoglycaemia and weight gain. Always discuss the options with the person with diabetes when goal setting, but always explain the risk to their long-term health if glycaemic control is not achieved in the first 2–5 years after diagnosis.
REFERENCES
1.Diabetes UK. Facts and Stats, 2016. https://www.diabetes.org.uk/Documents/Position statements/DiabetesUK_Facts_Stats_Oct16.pdf
2. Adler AI, Erqou S, Lima TA, Robinson AH. Association between glycated haemoglobin and the risk of lower extremity amputation in patients with diabetes mellitus – review and meta-analysis. Diabetologia 2010;53:840–9
3. UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. (UKPDS 33) Lancet 1998;352: 837–53
4. Chalmers J, Cooper M. UKPDS and the Legacy Effect. N Eng J Med 2008;359:1618–20
5. Khunti K, Wolden ML, Thorsted BL, et al. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care 2013;36(11):3411–7
6. Aujoulat I, Jacquemin P, Rietzscel E, et al. Factors associated with clinical inertia: an integrative review. Adv Med Educ Pract 2014;5:141–7
7. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–149
8.Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589
9. NICE. Type 2 diabetes in adults: management [NG28], 2015 (updated 2017). https://www.nice.org.uk/guidance/ng28
10. NICE. Diabetes in adults (QS6). https://www.nice.org.uk/guidance/qs6/uptake.
11. Evans JL, Rushakoff R (2010) Oral Agents, Incretins and other "Non-Insulin" Pharmacologic Interventions for Diabetes. http://diabetesmanager.pbworks.com/w/page/17680289/Oral%20Pharmacological%20Agents%20for%20Type%202%20Diabetes
12. Epidemiology of Diabetes Interventions and Complications Study (EDIC) Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy. JAMA 2003;290(16):2159–67
13. ACCORD Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59