GLP1 receptor agonists – back in the premier division!
Beverley Bostock-Cox
Beverley Bostock-Cox
RGN MSc MA QN
Nurse Practitioner Mann Cottage Surgery, Moreton in Marsh
Education Lead, Education for Health, Warwick
The management of diabetes has evolved beyond the ‘take your pick’ approach of the older NICE guidelines, with the choice of drug guided by individual patient characteristics. Here we review the evidence for the glucagon-like peptide-1 receptor agonists
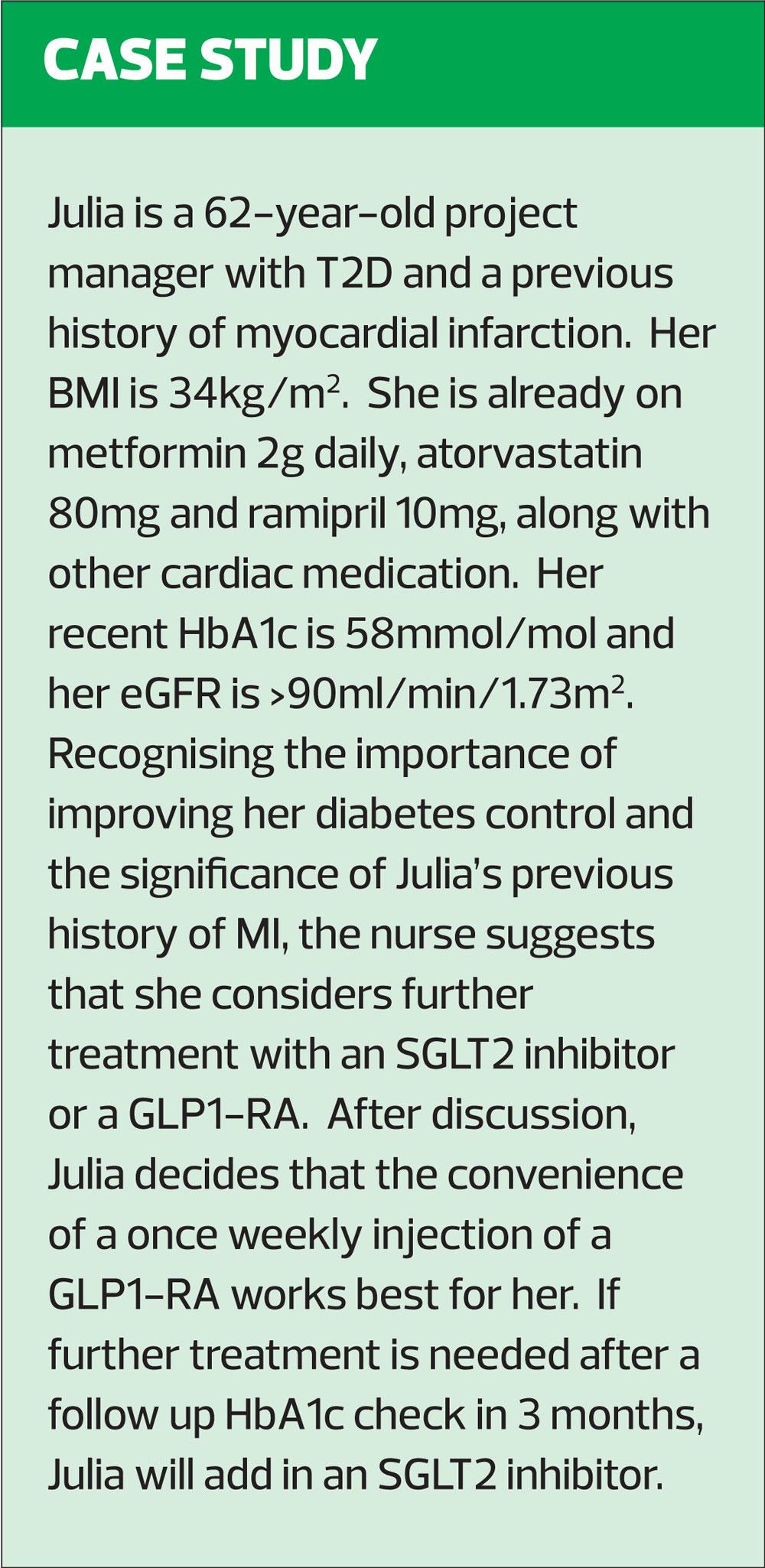
In 2015, the National Institute for Health and Care Excellence (NICE) published its guidance on the management of type 2 diabetes (T2D).1 The section on pharmacological interventions for glycaemic control recommended metformin first line, unless contraindicated, in line with the more recent international guidance from the America Diabetes Association (ADA) and Europe Association for the Study of Diabetes (EASD).2 With NICE, the decision as to what to use next after metformin was left to the clinician, but the glucagon-like peptide-1 receptor agonists (GLP1-RAs) were mainly reserved for use when other non-insulin therapies had failed to ensure that the patient achieved good glycaemic control. NICE guidance was updated in 2017 and is currently being updated again. It is anticipated that the updated guideline may well suggest that GLP1-RAs or sodium glucose reuptake transporter inhibitors (SGLT2i) should be considered as the next option after metformin in most cases. The ADA/EASD guidelines already advise along these lines.2
In this article, the role of GLP1-RAs will be considered in light of more recent evidence which has put them back into the spotlight. By the end of this article you will be able to:
- Recognise the mode of action of GLP1-RAs
- Have a better understanding of when they should be used
- Evaluate the advantages and disadvantages of different GLP1-RAs
- Analyse the benefits of GLP1-RAs beyond glycaemic control
THE MODE OF ACTION OF GLP1-RAs
GLP1-RAs (along with dipeptidyl peptidase-4 [DPP4] inhibitors such as sitagliptin and linagliptin) are known as incretin based therapies. Incretin hormones, including GLP-1, rise intermittently during the day when food is consumed and help to control blood glucose levels by increasing the amount of insulin produced in the beta cells of the pancreas on a glucose-dependent (i.e. ‘as required’) basis. GLP-1 also lowers glucagon secretion from the alpha cells of the pancreas, reducing the amount of glucose produced by the liver, which also helps to lower blood glucose levels.3 Once glycaemic stability has been achieved, the glucose-dependent mechanism of GLP1-RAs means that levels will not continue to drop, which is why these therapies are not associated with hypoglycaemia. GLP1-RAs are injectable therapies, which can be given once weekly, once daily or twice daily, depending on the product.
GLP1-RA therapies have benefits in addition to glycaemic control as they increase feelings of satiety (fullness) and reduce appetite which can impact on food intake and potential weight loss, both of which can support improved blood glucose levels.4 There is also evidence that they can reduce systolic blood pressure,5 which is an advantage in the population with T2D where around 75% of individuals have hypertension requiring treatment.6
The main side effects of GLP-1 RA drugs are usually transient and mild and include nausea and dyspepsia resulting from the slowing of gastric emptying. Although there have been concerns about a possible increased risk of pancreatitis and carcinoma of the pancreas or thyroid in incretin therapy users, both the USA Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have stated that evidence shows no statistically increased rates of these conditions in people taking DPP-4 inhibitors or GLP-1 therapies. A summary of key study findings can be found here http://www.glucagon.com/GLP-1AdverseEvents.html. However, caution should be used in people with a previous history of pancreatitis.
HOW SHOULD THEY BE USED?
Current GLP1-RA therapies are licensed to be used at every stage of the diabetes medication algorithm. However, the ADA/EASD guidelines from 2018 suggest that they should be considered as a key second line option after metformin for people who have:
- A history of cardiovascular disease
- A weight problem
- The need to avoid hypoglycaemia
- Chronic kidney disease or heart failure
The ADA/EASD guidelines have, therefore, identified patient features that will help clinicians to tailor treatment more effectively in order to improve overall outcomes. The reason for suggesting GLP1-RAs for these categories is as follows:
A history of cardiovascular disease
There is evidence to suggest that some GLP1-RAs may be associated with improved cardiovascular outcomes, an important consideration for people with diabetes who are at increased risk of cardiovascular disease. This is discussed in more detail below.
A weight problem
As described above GLP1-RAs can help people to lose weight by increasing the sensation of satiety and regulating appetite.
The need to avoid hypoglycaemia
The glucose-dependent mechanism of GLP1-RAs means that they are unlikely to cause hypoglycaemia.
Chronic kidney disease and heart failure
GLP1-RAs can be used in chronic kidney disease (licences differ) and some have been shown to lead to improved renal outcomes in people with diabetes. Once again, this is important when people who have diabetes are at increased risk of renal impairment, end stage renal disease (ESRD) and dialysis.7
ADA/EASD also suggests GLP1-RAs as drugs that can be used in patients with diabetes and heart failure, although at the moment this is primarily based on the likelihood that they do no harm, rather than providing explicit benefit. Conversely, some of the SGLT2 inhibitors have shown evidence of benefit so these would take priority over GLP1-RAs until any further trials are completed.8
These additional benefits beyond glycaemic control vary from product to product.
THE SIMILARITIES AND DIFFERENCES BETWEEN DIFFERENT GLP1-RAs
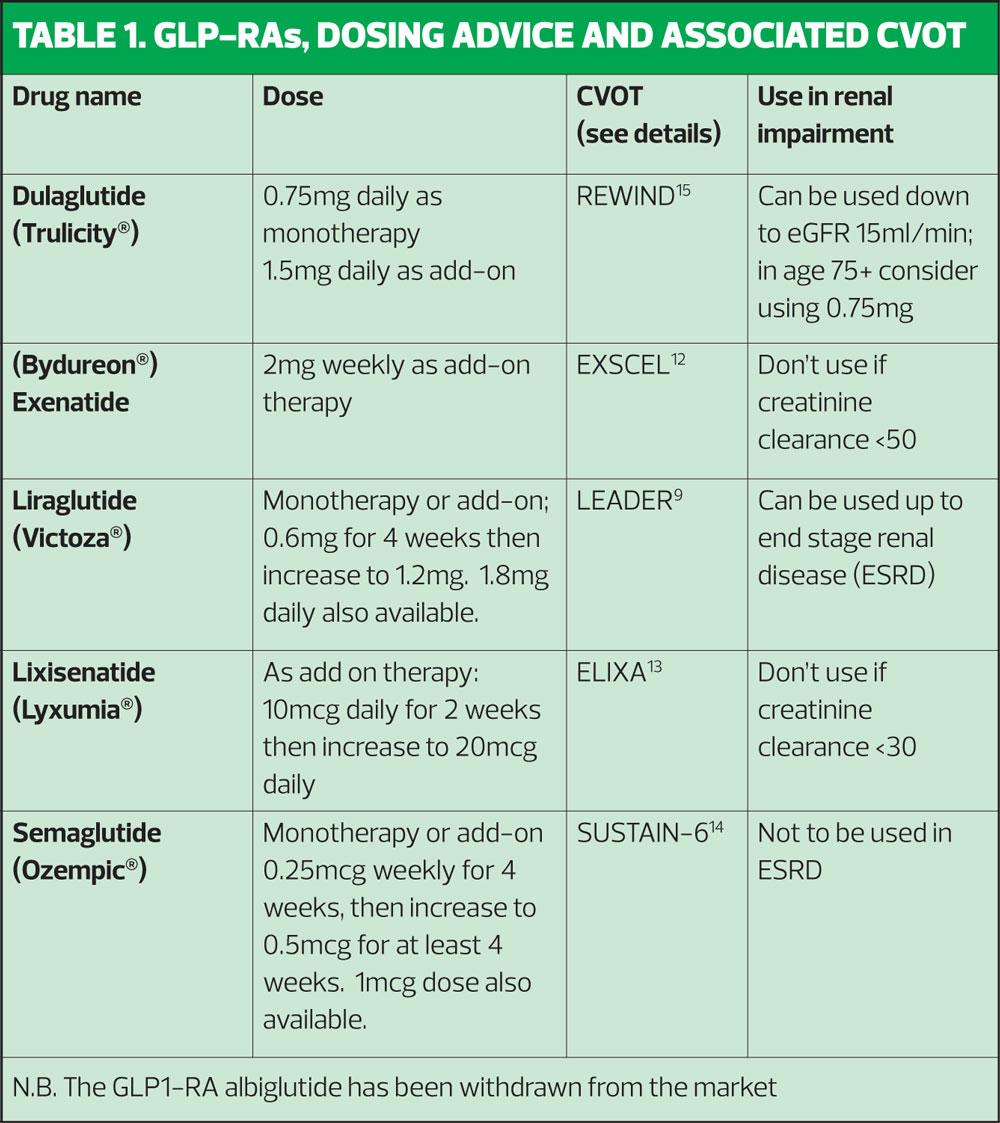
Currently available GLP1-RA products include weekly and daily options along with two GLP1-RA/insulin combinations. Table 1 shows the different GLP1-RAs available along with dosing advice and the key cardiovascular outcomes trial (CVOT) associated with the product. A summary of product characteristics for each product can be found at https://www.medicines.org.uk/emc/
Consideration should be given to whether the GLP1-RA is to be used as monotherapy (i.e. if metformin is contraindicated or not tolerated) or as an add-on therapy, the frequency of dosing, any co-existing conditions such as cardiovascular or renal disease and the injection device for each product. General practice nurses (GPNs) should have access to placebo devices so they can show patients the different types of device and teach correct injection technique, including infection control and sharps management, before the decision is made to prescribe.
GLP1-RA AND INSULIN COMBINATIONS
There are currently two licensed GLP1-RA/insulin combination preparations available to prescribe in the UK. These are Xultophy® (liraglutide and insulin degludec) and Suliqua® (lixisenatide and insulin glargine – Lantus).
These combinations of a GLP-1 RA and basal insulin provide two different approaches to managing glycaemia. Using a GLP1-RA with insulin provides extra advantages beyond improved glycaemic control, such as weight loss, reduced risk of hypoglycaemia and potential cardiovascular benefits, as seen with liraglutide in the Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes (LEADER) trial.9 Using a combination product may also help to reduce the number of injections required, which is often preferred by the patient, as well as reducing possible side effects from insulin injections such as lipodystrophy.10
THE POSITION OF GLP1-RAs IN CURRENT NATIONAL AND INTERNATIONAL GUIDELINES
Following the blacklisting of rosiglitazone as a result of an observed increase in cardiovascular events in those using it, a requirement for proof of cardiovascular safety was introduced. Diabetes drugs developed since then must now demonstrate specific evidence of cardiovascular safety. However, more recent trials have gone beyond simple safety assurances and have begun to look at whether there may be any advantages to using newer classes of drugs, particularly with respect to cardiovascular outcomes. These cardiovascular outcome trials (CVOTs) are helping to develop the management of diabetes beyond the ‘take your pick’ approach of the older NICE guidelines. The ADA/EASD consensus recommendations state that decisions about how to optimise medication for glycaemic control will depend on the patient profile.2 This is because, as indicated above, newer drugs such as GLP1-RAs and SGLT2 inhibitors are showing benefits beyond glycaemic control.
Previously, cost implications may have impacted on decisions regarding whether or not to prescribe these drugs but the reality is that when £1 in every £10 spent by the NHS is on diabetes care, and 80p of that £1 is spent on treating the complications of diabetes, drugs such as GLP1-RAs and SGLT2 inhibitors should be seen as an investment, reducing some of the key complications associated with diabetes and therefore long-term costs.4,5,8
THE BENEFITS OF GLP1-RAs BEYOND GLYCAEMIC CONTROL
LEADER (the liraglutide and cardiovascular outcomes trial) recruited 9,340 patients who were followed up for a median 3.8 years.9 Subjects had T2D and were at high risk of a cardiovascular event. The primary composite outcome was the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke.
The event rate was significantly lower in the liraglutide group (13.0% v 14.9%, hazard ratio [HR] 0.87; 95% confidence interval [CI], 0.78 to 0.97; P<0.001 for noninferiority; P=0.01 for superiority). All-cause mortality was also significantly reduced. Some commentators have noted that the impact of liraglutide on CV death and all-cause mortality did take longer to be seen than the similar outcomes noted in EMPA-REG, the CVOT using the SGLT2 inhibitor empagliflozin. CV benefits were seen at around 12-18 months, compared with less than 3 months with empagliflozin in EMPA-REG.11 However, positive changes were seen in a range of specific cardiometabolic risk factors including HbA1c, systolic blood pressure and weight.
Of note is the fact that a similar trial with once-weekly exenatide did not show evidence of benefit, although the safety signal was reassuring. In the EXSCEL study, where once weekly exenatide was used in patients with T2D with or without previous cardiovascular disease, the incidence of major adverse cardiovascular events did not differ significantly between patients on treatment versus those who received placebo. However, small but positive changes were still observed in the same parameters: HbA1c, systolic BP and weight.12
ELIXA studied the effect of using lixisenatide in people with recent acute coronary syndrome. No cardiovascular benefits were shown, although small but positive and statistically significant improvements were again seen in the HbA1c, systolic BP and weight.13
SUSTAIN-6 used semaglutide in 3,297 high-risk patients, of whom 83% had established cardiovascular disease, including chronic kidney disease, and showed a 26% reduction in the composite primary endpoint of cardiovascular death, nonfatal myocardial infarction and nonfatal stroke. Again, positive changes were observed in HbA1c, systolic BP and weight.14
REWIND enrolled 9,901 participants to receive dulaglutide or placebo in addition to their other diabetes medication and were followed up for 5.4 years, making this the longest trial of a GLP1-RA in a CVOT. Just over a third of the population did not have a history of CVD, making this a much broader diabetes population. The primary outcome occurred in 594 (12.0%) participants in the treated group and in 663 (13.4%) participants in the placebo group (HR, 0·88, 95% CI 0·79–0·99; P=0·026), suggesting a potential benefit. All-cause mortality did not differ between groups.15
It is important to consider differences in trial length, population and inclusion/ exclusion criteria that might influence outcomes.
These results indicate that although GLP1-RAs may be effective at reducing HbA1c and also seem to have small but positive effects – for the most part – on blood pressure and lipids, it is not possible to assume a class effect when it comes to CV benefits, although it may also be that the trial populations and design impacted on results. However, the molecular structure of the drugs is different and the length of action and impact on glycaemic control varies, too.
GLP1-RAs AND RENAL OUTCOMES
Renal impairment, including end stage renal disease is a major concern in the management of diabetes and has a significant impact on patients, their families and resources. It is estimated that the number of people who need dialysis (which is very costly, has a negative impact on quality of life and has a poor prognosis) is set to rise from 3 million to 5 million worldwide by 2035, fuelled by the increasing prevalence of diabetes. With regard to the potential renoprotective benefits of GLP1-RA drugs, there is developing evidence.
Secondary renal outcomes measured in the AWARD-7 trial using dulaglutide, showed that after one year, eGFR was higher in people with T2D and moderate/severe CKD in the treated group than in people on insulin glargine and that an early increase in eGFR in the treated group had been observed.16 More than 90% of the patients in AWARD-7 were already being treated with renin–angiotensin system blockade drugs such as ACE inhibitors and angiotensin receptor blockers. There were also some positive findings for renal disease in LEADER, EXSCEL, ELIXA and SUSTAIN-6, particularly in terms of reductions in macroalbuminuria.
INTERNATIONAL RECOMMENDATIONS
The ADA/EASD guidelines state that:
- Among patients with type 2 diabetes who have established atherosclerotic cardiovascular disease, (ASCVD), GLP-1 receptor agonists or SGLT2 inhibitors with proven cardiovascular benefit are recommended as part of glycaemic management
- Among patients with ASCVD in whom HF coexists or is of special concern, SGLT2 inhibitors are recommended and GLP1-RAs may be considered
- For patients with T2D and chronic kidney disease (CKD), with or without CVD, a GLP-1 receptor agonist shown to reduce CKD progression can be considered if an SGLT2 inhibitor is contraindicated or declined.
Other recommendations from ADA/EASD state that the choice of glucose-lowering medication if there is a compelling need to minimise weight gain or promote weight loss should focus on an SGLT2 inhibitor or a GLP1-RA.
When choosing glucose-lowering medication in a situation where there is a compelling need to minimise hypoglycaemia, ADA/EASD guidelines state that a GLP1-RA may be an appropriate choice.
SUMMARISING THE ROLE OF GLP1-RAs IN T2D
GLP1-RA therapies are injectable therapies that improve glycaemic control. Some of the drugs in this class have been shown to have a positive impact on cardiovascular and renal outcomes as well as on specific parameters such as weight, blood pressure and lipid profiles. The choice of which GLP1-RA to use will depend on the individual patient profile, including the history of CVD or presence of CV risk factors, along with decisions about frequency of injections.
REFERENCES
1. NICE NG28. Type 2 diabetes in adults: management (2015, updated 2017) https://www.nice.org.uk/guidance/ng28/resources/type-2-diabetes-in-adults-management-pdf-1837338615493
2. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41(12):2669-2701 https://care.diabetesjournals.org/content/41/12/2669
3. Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 2008;60(4):470–512. doi:10.1124/pr.108.000604
4. Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context 2015;4:212283. doi:10.7573/dic.212283
5. Boyle JG, Livingstone R, Petrie JR Cardiovascular benefits of GLP-1 agonists in type 2 diabetes: a comparative review. Clinical Science 2018;132(15):1699-1709;DOI: 10.1042/CS20171299
6. Khangura DS, Waqar Salam M, Brietzke SA, et al. Hypertension in Diabetes. [Updated 2018 Feb 14]. In: Feingold KR, Anawalt B, Boyce A, et al (Eds). Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000. https://www.ncbi.nlm.nih.gov/books/NBK279027/
7. Morrish NJ, Wang SL, Stevens LK, et al. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia 2001;44(2):14–21
8. Del Olmo-Garcia MI, Merino-Torres JF. GLP-1 Receptor Agonists and Cardiovascular Disease in Patients with Type 2 Diabetes. J Diabetes Res 2018;4020492. doi:10.1155/2018/4020492
9. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2016; 375:311-322 DOI: 10.1056/NEJMoa1603827
10. Gentile S, Strollo F, Ceriello A. Lipodystrophy and Associated Risk Factors in Insulin-Treated People With Diabetes. Int J Endocrinol Metab 2016;14(2): e33997. doi:10.5812/ijem.33997
11. Zinman B, Wanner C, Lachin JM, et al for the EMPA-REG OUTCOME Investigators. N Engl J Med 2015;373(22):2117-28.
12. Holman RR, Bethel MA, Mentz RJ, et al, for the EXSCEL Study Group. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–1239 pmid:28910237
13. Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators . Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–225
14. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 pmid:27633186
15. Gerstein HC, Colhoun HM, Dagenais GR et al. Dulaglutide and cardiovascular outcomes (REWIND) The Lancet. ePub 9 June 2019. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)31149-3/fulltext
16. Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol 2018; 6: 605-617