Choosing sodium glucose cotransporter 2 inhibitors: examining the evidence
Beverley Bostock-Cox
Beverley Bostock-Cox
RGN MSc MA QN
Nurse Practitioner Mann Cottage Surgery, Moreton in Marsh
Education Lead, Education for Health, Warwick
As in any prescribing decision, the benefits and risks of any class of drug treatment should be discussed, and treatment choices based on effectiveness, safety and tolerability, and the needs of the individual. And when choosing within class there are differences in the evidence base to help guide decision-making
In October 2018, the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) glycaemic lowering guidelines were updated to include evidence from recent trials. In this update, prominence was given to the use of sodium glucose cotransporter 2 inhibitors (SGLT2i) as a second line therapy after metformin, for people with a history of cardiovascular disease, chronic kidney disease or clinical heart failure and atherosclerotic cardiovascular disease.1 The NICE guidelines on diabetes were published some time before, in 2015, but were updated in 2017 to reflect the increasing importance of this class of drugs in the management of diabetes.2 There are three SGLT2i drugs currently available on the market, with another due to launch in 2019. In this article the evidence for individual SGLT2 inhibitors is analysed, with a particular focus on the additional benefits of these glucose-lowering drugs.
By the end of this article you should be able to:
- Recognise the role for SGLT2i drugs in the management of type 2 diabetes
- Evaluate their impact on cardiovascular and renal disease
- Analyse the key cardiovascular outcomes trials
- Consider the effect of SGLT2i drugs on renal function
SGLT2 INHIBITORS – MECHANISM OF ACTION
The mechanism of action of SGLT2i drugs is unique. In the kidneys, SGLT2 receptors ensure that glucose in the bloodstream is reabsorbed from the renal system back into the body. SGLT2 inhibitors impede this action, allowing glucose to be excreted via the urine instead of being reabsorbed. The resulting glycosuria means that glycaemic levels are reduced and weight loss is achieved through the loss of calories, while the mild osmotic effect also reduces blood pressure. Microvascular endothelial benefits are also achieved.3 It is thought that this is part of the reason why this class of drugs has shown cardiovascular benefits, including improvements in renal outcomes, in recent trials.
IMPACT ON CARDIOVASCULAR OUTCOMES
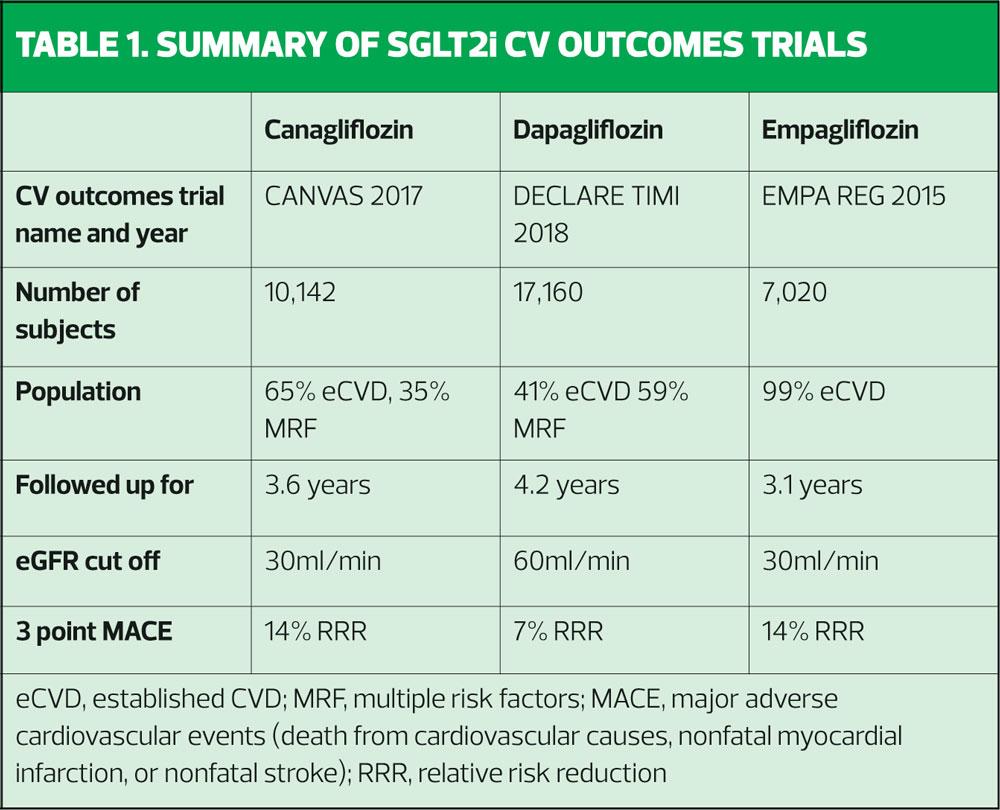
There are three major cardiovascular outcomes studies. These are, in the order in which they were published, EMPA REG,4 Canagliflozin Cardiovascular Assessment Study (CANVAS),5 and the Dapagliflozin Effect on CardiovascuLAR Events – Thrombolysis In Myocardial Infarction 58 (DECLARE-TIMI 58).6 These are summarised in Table 1.
EMPA REG
The EMPA REG study, published in 2015, recruited over 7,000 patients and followed them up for an average of just over 3 years.4 This study focused on adults with type 2 diabetes (T2D) who already had established cardiovascular disease. In other words, these patients were very high risk, having already had a cardiovascular event (CVE). Another feature of the study group which made them high risk, was the fact that they could still take part even if their estimated glomerular filtration rate (eGFR) was as low as 30ml/min/1.73 m2. In EMPA REG, participants had to have had no hypoglycaemic agents for at least 12 weeks before randomisation and had to have an HbA1c of between 53mmol/mol and 75mmol/mol. Alternatively, they were included if they had had stable glucose-lowering therapy for at least 12 weeks before randomisation with an HbA1c between 53mmol/mol and 86mmol/mol. All received standard care and were then randomised to empagliflozin 10mg, increasing to 25mg where indicated, or placebo. The primary outcome measured was major adverse cardiovascular events (MACE) which includes death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. The results showed that MACE occurred in 490 of 4687 patients (10.5%) in the active treatment group and in 282 of 2333 patients (12.1%) in the placebo group (hazard ratio [HR], 0.86; 95.02% confidence interval [CI], 0.74–0.99; P=0.04 [i.e. statistically significant for superiority]). In the empagliflozin group there were significantly lower rates of death from cardiovascular causes (3.7%, vs. 5.9% in the placebo group; 38% relative risk reduction), hospitalisation for heart failure (2.7% and 4.1%, respectively; 35% relative risk reduction), and death from any cause (5.7% and 8.3%, respectively; 32% relative risk reduction). Thus empagliflozin was the first SGLT2i to show that people with T2D and cardiovascular disease (CVD) had a lower rate of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke and of death from any cause if they were treated with this drug compared with those who did not receive it.
CANVAS
Two years later, in 2017, the Canagliflozin Cardiovascular Assessment Study – CANVAS – was published.5 In line with EMPA REG, this randomised controlled trial was designed to evaluate the impact of canagliflozin (100-300mg) versus placebo on the incidence of cardiovascular death, myocardial infarction, or stroke. However, the study population was different from that recruited to EMPA REG (and DECLARE-TIMI 58, as seen below) because one third of subjects had multiple risk factors for CVD rather than having an actual history of CVD, as was the case in EMPA REG. The other two thirds already had known CVD. CANVAS was a bigger study than EMPA REG, recruiting over 10,000 patients. The impact of canagliflozin on the prevention of CVD was evaluated by including people aged 30 years or more with a history of symptomatic atherosclerotic cardiovascular disease, and through the inclusion of people age 50 or above with at least two risk factors for CVD. These risk factors included having T2D for more than 10 years, (the average duration of diabetes was 13.5 years), having a systolic blood pressure of more than 140 mmHg on antihypertensive therapy, current smoking, the presence of albuminuria, or having a high-density lipoprotein (HDL) cholesterol of less than 1mmol/l. The average age of patients in the study was 63 and just over a third were women. Follow up was for 188 weeks. CANVAS demonstrated a statistically significant reduction in the primary outcome of incidence of cardiovascular death, myocardial infarction, or stroke, in the patients treated with canagliflozin, when compared with the placebo group.
The benefits of canagliflozin were seen across both primary and secondary prevention groups but were most marked in people with a history of CVD.
Interestingly, the benefits of canagliflozin appeared to be greater among people with a prior history of heart failure. It has been postulated that this is because of the reabsorption of sodium and glucose which occurs with SGLT2i drugs, and which leads to a mild osmotic diuretic effect and natriuresis, which will be beneficial in people with heart failure. These effects have also been linked to the clinically significant reductions in both systolic and diastolic blood pressure (reductions of 2.46 mmHg and 1.46 mmHg respectively) which have been seen in studies with SGLT2i therapies along with reductions in total cholesterol and triglyceride levels.6,7 The cardiovascular benefits of canagliflozin were maintained irrespective of renal function.5
DECLARE TIMI
In 2018, the DECLARE-TIMI trial was published, focusing on the third SGLT2i currently on the market in the UK, so examining the effect of dapagliflozin on MACE.6 The number of participants in DECLARE TIMI was the highest of all three trials, with a total of 17,160 recruits, who were followed up for an average of 4.2 years. The group was a mixed one, like CANVAS, with people who already had CVD included, along with those who did not have a history of CVD but who had CVD risk factors. A key difference between DECLARE TIMI compared with EMPA REG and CANVAS was that people with an eGFR of less than 60ml/min were excluded from the study. This is likely to have an effect on the risk profile of the group, bearing in mind that renal impairment is a risk factor for CVD. The results of DECLARE TIMI showed that the primary composite outcome of hospitalisation for heart failure or cardiovascular death was significantly reduced in the dapagliflozin group compared with the placebo group, with a relative risk reduction of 17% in patients with or without established CVD or heart failure. This effect was on top of the standard of care for CV risk management.
Of real interest in this study was the fact that the biggest contributory factor to this outcome was the 27% relative risk reduction in hospitalisation for heart failure in a population where the majority of people had no history of heart failure. It has been postulated that the reason for this important outcome is, in part, the diuretic effect of the SGLT2i group and the added benefit of the drop in blood pressure which has been seen in these trials. However, there was no significant statistical difference between major adverse cardiac events in the treatment versus placebo group in DECLARE TIMI and as discussed, a possible reason for this is that only those with normal renal function (eGFR > 60ml/min) were allowed into the study, meaning that these were at lower overall risk of CVD events.
RISKS ASSOCIATED WITH SGLT2 INHIBITORS
In a study where the use of SGLT2 inhibitors was compared with GLP1 receptor agonists, an increased risk of lower limb amputation was identified (incidence rate 2.7 v 1.1 events per 1,000 person years, hazard ratio [HR] 2.32, 95% confidence interval [CI] 1.37 – 3.91) and diabetic ketoacidosis (1.3 v 0.6, HR 2.14, CI 1.01 – 4.52) although actual numbers, and therefore events, were small.10 There were no associations between SGLT2i therapy and bone fracture (15.4 v 13.9, HR 1.11, CI 0.93 – 1.33), acute kidney injury (2.3 v 3.2, HR 0.69, CI 0.45 – 1.05), serious urinary tract infection (5.4 v 6.0, HR 0.89, CI 0.67 – 1.19), venous thromboembolism (4.2 v 4.1, HR 0.99, CI 0.71 – 1.38) or acute pancreatitis (1.3 v 1.2, HR 1.16, CI 0.64 – 2.12) in this study – adverse drug reactions to SGLT2 inhibitors that have been included in their summaries of product characteristics, based on previous findings. However, recent research has suggested a possible increased risk of cholangioma in people using incretin based therapies (DPP4 inhibitors and GLP1 mimetics) so as always, it is important to weigh up the risk: benefit ratio with every therapeutic option.11
In DECLARE TIMI there was no imbalance between the active and placebo groups when it came to lower limb amputation or fracture.
Overall, then, it would seem that the evidence of benefit of SGLT2i therapy with respect to glycaemic control, reduction of MACE risk, particularly in people with established CVD, significant reductions in hospitalisations for heart failure and potential benefits for blood pressure, lipids and renal function far outweighs any clear evidence of risk.
SO HOW DO WE CHOOSE?
The simple answer should be that we don’t, the patient should, and that this would only happen after careful consideration of the pros and cons, risks and benefits, and with respect to the bioethical principles of autonomy, beneficence, non-maleficence and justice. However, reality calls and there may be other issues which determine choice such as local formularies (caveat emptor) or even the arcane practice in some areas of insisting that people who might benefit from an SGLT2i should be referred to a specialist prior to initiation. When considering the fact that the EASD/ADA guidelines recommend these drugs second line, that is going to be a lot of referrals for a specialist opinion about a drug class which many people are going to need.
CONCLUSION
SGLT2 inhibitors have proven benefits for glycaemic control, reducing cardiovascular events and for their impact on renal impairment, although the latter has not yet been included in any product licence. When treating adults with type 2 diabetes, the benefits and risks of any drug treatment should be discussed and treatment choice should be tailored to each individual. Decisions as to which class is best suited to that individual may be based on the effectiveness of the drug overall, safety and tolerability and the individual’s own medical needs and social circumstances. When choosing within class there are arguably some differences in the evidence base which may help to guide decision making but the overriding message is that these drugs are a key therapeutic option when managing type 2 diabetes holistically.
REFERENCES
1. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018. https://doi.org/10.1007/s00125-018-4729-5 ePub: 05 October 2018
2. NICE NG28. Type 2 diabetes in adults: management, 2015, updated 2017 https://www.nice.org.uk/guidance/ng28
3. Sugiyama S, Jinnouchi H, Kurinami N, et al. The SGLT2 Inhibitor Dapagliflozin Significantly Improves the Peripheral Microvascular Endothelial Function in Patients with Uncontrolled Type 2 Diabetes Mellitus. Internal medicine (Tokyo, Japan) 2018;57(15): 2147-2156
4. Zinman B, Wanner C, Lachin JM, et al. EMPA-REG OUTCOME Investigators. N Engl J Med 2015;373(22):2117-28.
5. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644-657
6. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. Am Heart J;200:83-89
7. Mazidi M, Rezaie P , Gao HK, Kengne AP. E?ect of sodium glucose cotransport-2 inhibitors on blood pressure in people with type 2 diabetes mellitus: A systematic review and metaanalysis of 43 randomized cohort trials with 22 528 patients. J Am Heart Assoc 2017:6:e004007. doi:10.1161/JAHA.116.004007
8. Wanner C. EMPA-REG OUTCOME: the nephrologist's point of view Am J Med 2017;130:S63-S72
9. Tucker ME. Canagliflozin Renal Outcomes Study Is Halted Early for E?cacy. Medscape 2018 https://www.medscape.com/viewarticle/899424
10. Ueda P, Svanström H, Melbye M et al. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study. BMJ 2018;363:k4365 doi: 10.1136/bmj.k4365
11. Abrahami D, Douros A, Yin H et al. Incretin based drugs and risk of cholangiocarcinoma among patients with type 2 diabetes: population based cohort study BMJ 2018;363:k4880
Related articles
View all Articles