Choosing and using a GLP-1 receptor agonist: A Practice Nurse Quick Guide
Practice Nurse 2021;51(4)
Practice Nurse 2021;51(4)
While most general practice nurses will be familiar with the main classes of oral agents used in the management of type 2 diabetes (T2D), they may be less confident in choosing and using a glucagon-like peptide-1 receptor agonist (GLP-1 RA).
HOW DO THEY WORK?
GLP-1 RAs act by
- Stimulating the release of insulin
- Inhibiting the release of glucagon
- Slowing absorption of glucose into the blood stream
- Reducing appetite which may aid weight loss, which also helps to improve blood glucose levels.1,2
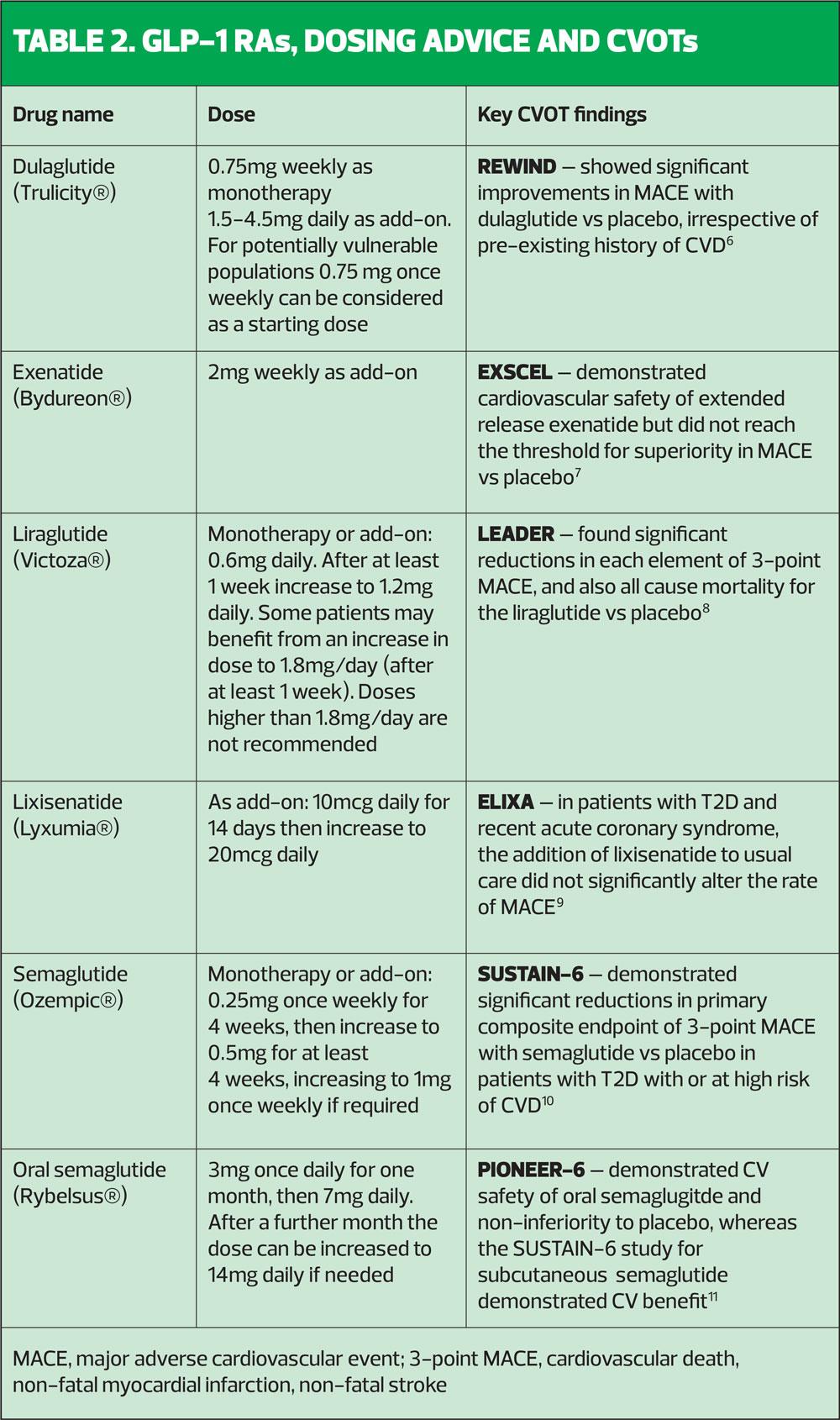
All GLP-1 RAs share the same underlying mechanism of action but differ in terms of formulations, administration, injection devices and doses. With six GLP-1 RAs currently available in the UK, each with its own characteristics, it is important that general practice nurses are familiar with these products so they can select the one that is most appropriate for the individual patient.3
The glucose-dependent mechanism of GLP-1 RAs means that they are not associated with hypoglycaemia.1
Most GLP-1 RAs (except oral semaglutide) are injected subcutaneously, and can be given once weekly, once daily or twice daily, depending on the product.
GLP-1 RAs are not indicated for use in type 1 diabetes
WHEN SHOULD THEY BE USED?
Current GLP-1 RAs are licensed to be used in type 2 diabetes as monotherapy or in addition to metformin (plus diet and exercise) or other agents when blood glucose is insufficiently controlled.
There is compelling evidence for the benefits of GLP-1 RAs, as well as SGLT2 inhibitors, in improving cardiovascular outcomes.4,5
The American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) consensus guideline recommends GLP-1 RAs with proven CVD benefits:
- For patients with T2D and established atherosclerotic cardiovascular disease (CVD)
- To reduce the risk of major adverse cardiovascular events (MACE) in patients without established CVD but with indicators of high CVD risk – patients aged ≥55 years with coronary, carotid or lower extremity stenosis >50%, left ventricular hypertrophy, eGFR <60 ml/min/1.73m2, or albuminuria.5
GLP-1 RAs are also recommended in patients with a compelling need to minimise hypoglycaemia, minimise weight gain or promote weight loss.5
ADVERSE EFFECTS AND PRECAUTIONS
GLP-1 RAs are general well tolerated. The most common adverse effects are nausea and vomiting, which are usually transient, and mild or moderate. These effects usually get less pronounced with time.3
- The frequency of nausea ranges from 13% with dulaglutide (0.75 mg) to 36% with once-daily exenatide.3
- Injection site reactions may occur in between 1% and 5.4%, depending on the preparation, dose and frequency of administration.3
- Dehydration (sometimes leading to acute renal failure or worsening of renal impairment) has been reported, usually in association with gastrointestinal side effects but sometimes alone, and patients should be advised to maintain fluid intake.3
- In clinical trials, acute pancreatitis has been observed, and patients should be advised of its characteristic symptoms – persistent, severe abdominal pain – and if suspected, GLP-1 RAs should be discontinued.3
Dose adjustment
- Obesity: No dose adjustment is needed based on body weight or body mass index.3
- Elderly: Most GLP-1 RAs do not require dose adjustment in elderly patients but a lower starting dose of dulaglutide (0.75 mg once weekly) can be considered in patients aged 75 years or older.
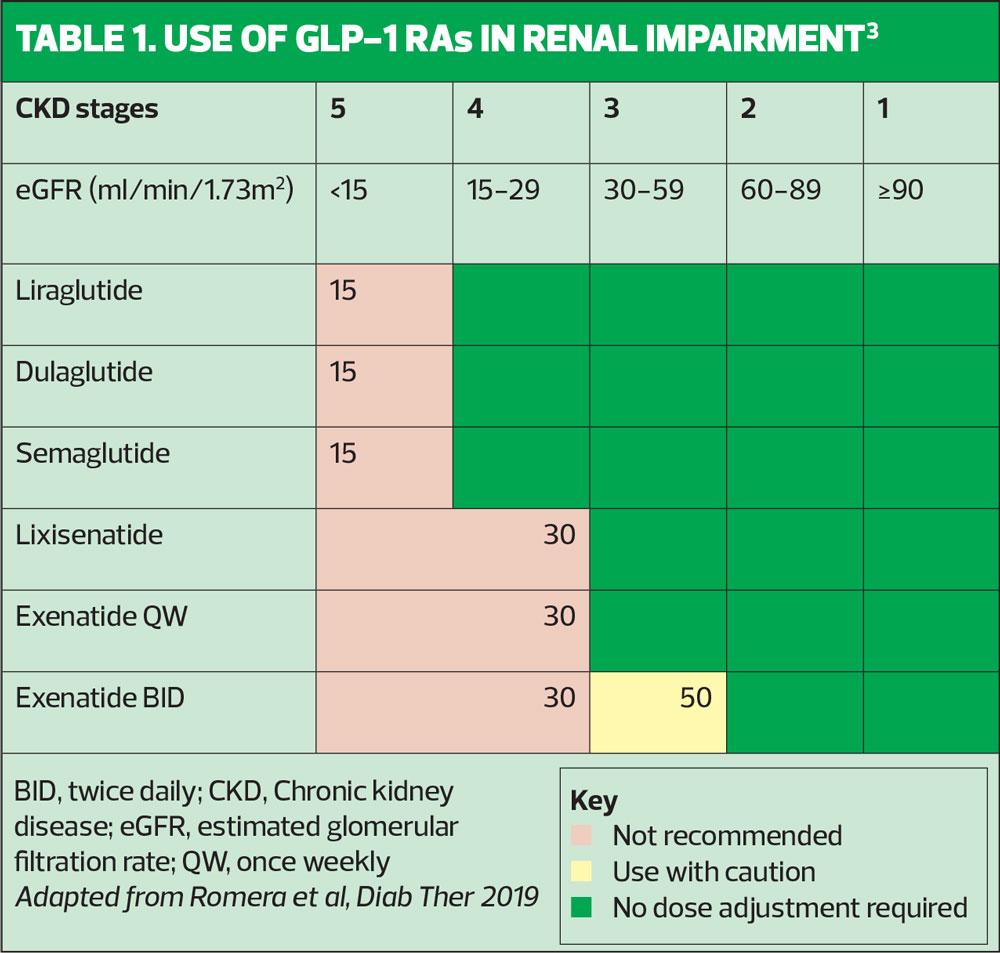
- Renal impairment: With the exception of exenatide, all GLP-1 RAs can be used without dose adjustment in in patients with mild or moderate renal impairment (Table 1).3
- Hepatic impairment: With the exception of liraglutide, all GLP-1 RAs can be used without dose adjustment in patients with hepatic impairment.3
BEFORE PRESCRIBING
- Consider the individual patient’s clinical characteristics, circumstances and preferences
- Consider potential side effects, impact on renal function and cardiovascular risk, and cost of the drug
- Consider the injection device and ease of use for the patient
- Consult the summary of product characteristics for each drug before prescribing
This Practice Nurse Quick Guide has been sponsored by Eli Lilly and Company, who have had no input into the content but who have reviewed it for accuracy
Job code: PP-DG-GB-0794
Date of preparation: April 2021
References
1. Kim W, Egan JM. Pharmacol Rev 2008;60(4):470-512. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2696340/pdf/nihms86105.pdf
2. Prasad-Reddy L, Isaacs D. Drugs in Context 2015;4:212283 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4509428/pdf/dic-4-212283.pdf
3. Romera I, Cebrián-Cuenca A, Álvarez-Guisasola F, et al. Diab Ther 2019;10:5-19
4. Davies MJ, D’Alessio DA, Fradkin J, et al. Diabetes Care 2017;41:2669-2701
5. Buse JB, Wexler DJ, Tsapas A. Diabetes Care 2020;43(2):487-493
6. Gerstein HC, Colhoun HM, Dagenais GR, et al. Lancet 2019;394:121-30
7. Holman R, Bethel MA, Mentz RJ, et al. N Engl J Med 2017;377:1228-39
8. Marso SP, Daniels GH, Brown-Frandsen, et al. N Engl J Med 2016;375:311-322
9. Pfeffer MA, Claggett B, Diaz R, et al. N Engl J Med 2015;373:2247-57
10. Marso SP, Bain SC, Consoli A, et al. N Engl J Med 2016;375:1834-44
11. Husain M, Birkenfeld AL, Donsmark M, et al. N Engl J Med 2019;381:841-851