A deep dive into diabetes
Practice Nurse 2022;52(7): online only
Practice Nurse 2022;52(7): online only
Faculty members at the inaugural meeting of specialist nurses working in the closely linked specialties of heart failure, renal disease and diabetes (hFRenDs) presented a ‘deep dive’ into their respective specialties: this article focuses on the role of SGLT2 inhibitors which are indicated in heart failure, renal disease as well as more conventionally in type 2 diabetes
Prevalence of diabetes continues to rise in the UK, with T2DM accounting for approximately 90% of cases.1 Challenges for specialist nurses include budget limitations for training, succession planning and career progression. Obtaining study leave can be extremely difficult. However, a recent study indicated that the introduction of inpatient Diabetes Specialist Nurse (DSN) provision was associated with reduced length of stay in hospital and improved clinical care for people with T2D, demonstrating the value of DSNs at the front-line.2
UPDATED GUIDANCE
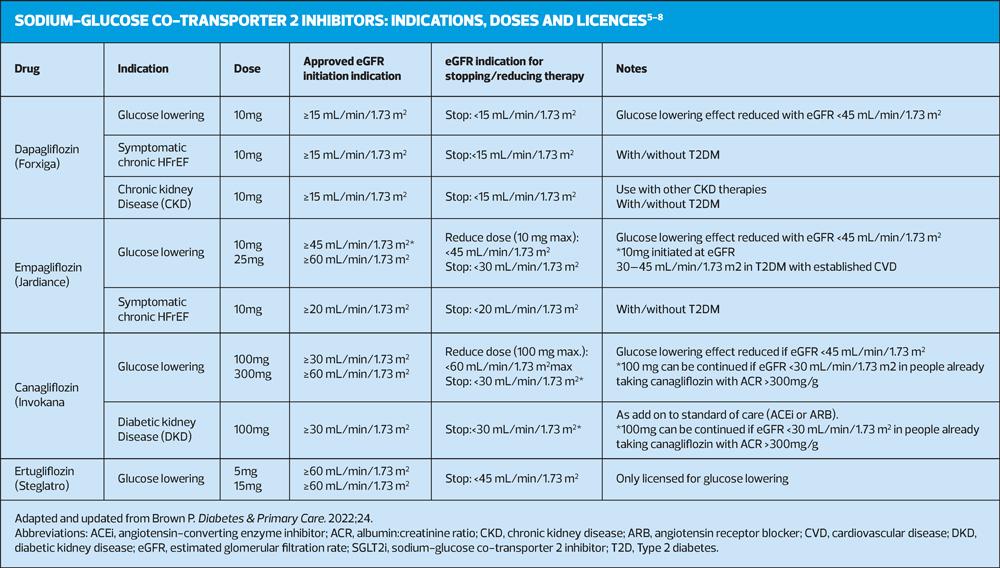
The NICE guidelines for the management of T2D in adults were updated in 2022 to incorporate recommendations regarding SGLT2i evidence and prescribing information, intercurrent illness management advice and symptoms and risk factors for diabetic ketoacidosis (DKA).3 The revised guidance recommends that people with high or increasing cardiovascular (CV) risk, or those who develop heart failure (HF) or atherosclerotic cardiovascular disease (ASCVD) should be offered an SGLT2i with proven CV benefit.3 SGLT2i therapy should also be offered to people with T2D who have failed to reach their target HbA1c.3 In recent years, the licence indication has changed for some of the available SGLT2is in response to emerging CV outcome trial (CVOT) data, with new indications for use in heart failure with reduced ejection fraction (HFrEF), chronic kidney disease (CKD) and diabetic kidney disease (DKD) approved in the UK (Table 2).4-7
SGLT2i treatments are still regarded as relatively new therapies, despite being available for the treatment of T2D for around 9 years.8-13 They inhibit SGLT2 protein activity within the proximal convoluted tubule in the kidney nephron to prevent reabsorption of glucose and increase its excretion in the urine.10 Via this mechanism, SGLT2is are able to reduce blood glucose levels independently of insulin or incretin pathways, lowering the risk of treatment-related hypoglycaemia.10 The glucose lowering effect observed in clinical practice is usually dependent on renal function. Treatment with SGLT2is is generally accompanied by weight loss.11-13
SICK DAY RULES
SGLT2i treatments are among those listed in the Diabetes UK ‘Sick Day’ or SADMAN recommendations due to their mode of action, which increases diuresis and can heighten the risk of dehydration during intercurrent illness.15,16
When a person with T2D is unwell, they must pause SGLT2i treatment until they are able to eat and drink normally again. 15,16 Individuals should be advised to replace meals with sugary fluids, if they are unable to eat or if they are vomiting, or eat easily digestible foods (e.g. soup, ice cream, yoghurts, milky drinks).15,16 When blood glucose levels are high, individuals should drink sugar-free fluids.15,16 In contrast with SGLT2is, insulin treatment does not need to be suspended during illness, although the dose may require adjustment.16 Blood glucose can rise, even when a person is not eating and levels should be monitored, with more frequent checks used where necessary.16 The prescribing information for current medications must be checked as some treatments will need to be given at higher doses.12
Circumstances when SGLT2i therapy should be temporarily suspended are typically when there is an elevated risk of dehydration:9,10,16
- Acute medical admission
- Admission for elective surgery or a procedure that requires starvation
- Vomiting
- Diarrhoea
Once the person is able to eat and drink again, they can resume treatment.9,14 In cases of diabetic ketoacidosis (DKA), SGLT2i treatment must be ceased unless there is evidence that it has been clearly resolved.9,15,16 DKA is relatively rare, occurring in approximately 1 in 1,000 to 1 in 10,000 people, but it is a serious complication and people at high risk should be monitored carefully.17,18 Risk factors for euglycaemic DKA include low beta cell function, which can occur in people with T2DM who have low c-peptide levels, in adults with latent autoimmune diabetes (LADA) or in those with a history of pancreatitis.19,20 Other risk factors are restricted food intake, severe dehydration, sudden reduction in insulin, increased insulin requirements due to acute illness, surgery and alcohol abuse.17,18 HCPs need to be aware of the signs and symptoms of DKA and patients must be counselled on when to seek urgent medical advice. A combination of the potential symptoms below may be present:17
- Glucose may be normal or only mildly raised (< 14 mmol/L)
- Nausea
- Vomiting
- Abdominal pain
- Deep and rapid breathing
- Drowsiness
- Sweet smelling breath (pear drops)
Blood ketone testing is required and levels of 3.0 mmol/L or more indicate the presence of DKA, with urgent care required.17
TYPE 2 DIABETES RESOURCES FOR PATIENTS AND CLINICAL STAFF
- NICE NG28. Type 2 diabetes in adults: management: https://www.nice.org.uk/guidance/ng28
- Diabetes UK Sick Day recommendations: https://www.diabetes.org.uk/guide-to-diabetes/life-with-diabetes/illness
- Su Down. How to advise on sick day rules: https://diabetesonthenet.com/diabetes-primary-care/how-to-advise-on-sick-day-rules/
- Pam Brown. Need to know guide: SGLT2 inhibitor indications, doses and licences: https://diabetesonthenet.com/diabetes-primary-care/sglt2i-indications-doses-licences/
- Jane Diggle. At a glance factsheet: Ketones and diabetes: https://diabetesonthenet.com/diabetes-primary-care/glance-factsheet-ketones-and-diabetes/
See also Nurses call for closer links across specialties, A deep dive into heart failure and A deep dive into renal failure
References
1. Diabetes UK. Diabetes Statistics. https://www.diabetes.org.uk/professionals/position-statements-reports/statistics
2. Akiboye F, Sihre HK, al Mulhem M, et al. Impact of diabetes specialist nurses on inpatient care: A systematic review. Diabetic Medicine 2021;38(9). doi:10.1111/dme.14573
3. NICE NG28. Type 2 diabetes in adults: management; Updated 29 June 2022. https://www.nice.org.uk/guidance/ng28
4. Brown P. Need to know guide: SGLT2 inhibitor indications, doses and licences. Diabetes & Primary Care 2022;24. https://diabetesonthenet.com/wp-content/uploads/Need-to-know_-SGLT2is_Feb-22.pdf
5. AstraZeneca UK Limited. Forxiga (dapagliflozin propanediol monohydrate) 10 mg tablets. Summary of product characteristics; 2022. https://www.medicines.org.uk/emc/product/7607/smpc
6. Napp Pharmaceuticals Limited. Invokana (canagliflozin hemihydrate) 100 mg and 300 mg tablets. Summary of product characteristics; 2022. https://www.medicines.org.uk/emc/product/8855/smpc
7. Boehringer Ingelheim Limited. Jardiance (empagliflozin) 10 mg and 25 mg tablets. Summary of product characteristics; 2022. https://www.medicines.org.uk/emc/product/5441/smpc
8. Merck Sharpe & Dohme (UK) Limited. Steglatro (ertugliflozin L-pyroglutamic acid) 5 mg and 15 mg tablets. Summary of product characteristics; 2022. https://www.medicines.org.uk/emc/product/9803/smpc
9. Dashora U, Gregory R, Winocour P, et al. Association of British Clinical Diabetologists (ABCD) and Diabetes UK joint position statement and recommendations for non-diabetes specialists on the use of sodium glucose co-transporter 2 inhibitors in people with type 2 diabetes (January 2021). Clin Med (Lond) 2021;21(3):204-210. doi:10.7861/clinmed.2021-0045
10. Kalra S. Sodium Glucose Co-Transporter-2 (SGLT2) Inhibitors: A Review of Their Basic and Clinical Pharmacology. Diabetes Ther. 2014;5(2):355-366. doi:10.1007/s13300-014-0089-4
11. Neeland IJ, McGuire DK, Chilton R, et al. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2016;13(2):119-126. doi:10.1177/1479164115616901
12. Bolinder J, Ljunggren Ö, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97(3):1020-1031. doi:10.1210/jc.2011-2260
13. Blonde L, Stenlöf K, Fung A, Xie J, Canovatchel W, Meininger G. Effects of canagliflozin on body weight and body composition in patients with type 2 diabetes over 104 weeks. Postgrad Med. 2016;128(4):371-380. doi:10.1080/00325481.2016.1169894
14. Brown P. How to use SGLT2 inhibitors safely and effectively. Diabetes & Primary Care. 2021;23(1):5-7.
15. Diabetes UK. Dealing with illness. Accessed July 19, 2022. https://www.diabetes.org.uk/guide-to-diabetes/life-with-diabetes/illness
16. Down S. How to advise on sick day rules. Diabetes Prim Care. 2020;22:47-48
17. Diggle J. Ketones and diabetes. Diabetes & Primary Care. 2020;22:49-50.
18. European Medicines Association. SGLT2 inhibitors: PRAC makes recommendations to minimise risk of diabetic ketoacidosis. Published 2016. Accessed July 19, 2022. https://www.ema.europa.eu/en/news/sglt2-inhibitors-prac-makesrecommendations- minimise-risk-diabeticketoacidosis
19. Diabetes UK. Latent autoimmune diabetes in adults (LADA). Accessed July 19, 2022. https://www.diabetes.org.uk/diabetes-thebasics/ other-types-of-diabetes/latent-autoimmunediabetes
20. Plewa MC, Bryant M, King-Thiele R. Euglycemic Diabetic Ketoacidosis. In: StatPearls [Internet]. Treasure Island. 2022. Accessed July 19, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554570/#_NBK554570_pubdet_
Related articles
View all Articles